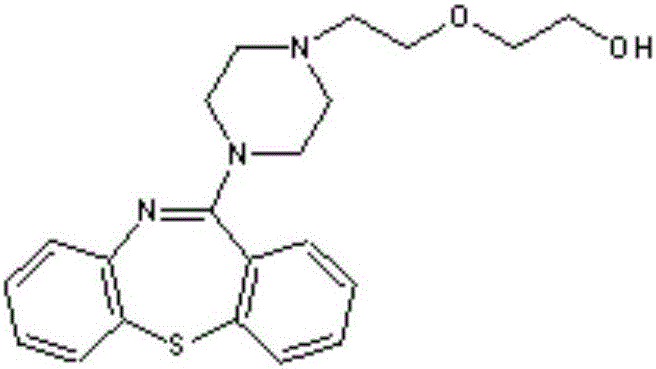
Quetiapine fumarate slow release pharmaceutical composition and preparation method thereof
A technology of quetiapine fumarate and sustained-release drugs, which is applied in the directions of drug combination, pharmaceutical formulation, drug delivery, etc., to achieve the effects of enhancing patient compliance, rapid onset of action, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] follow the prescription
[0039] Table 1 Specifications: 200mg
[0040]
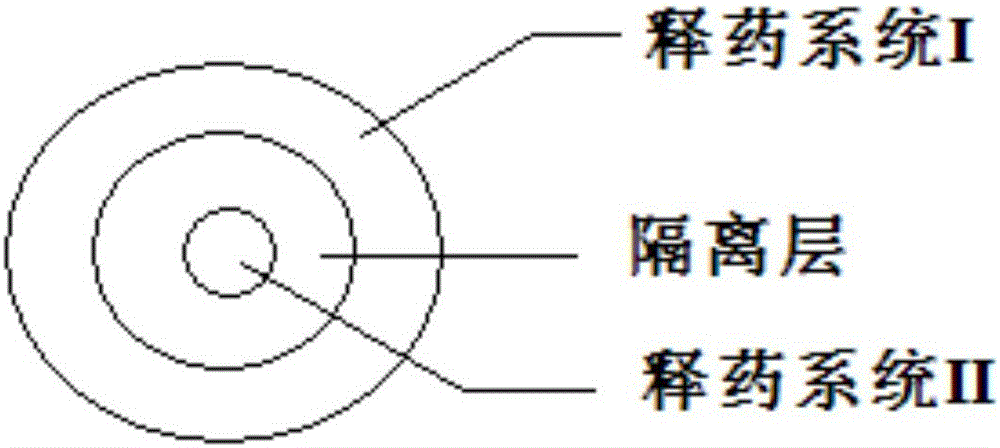
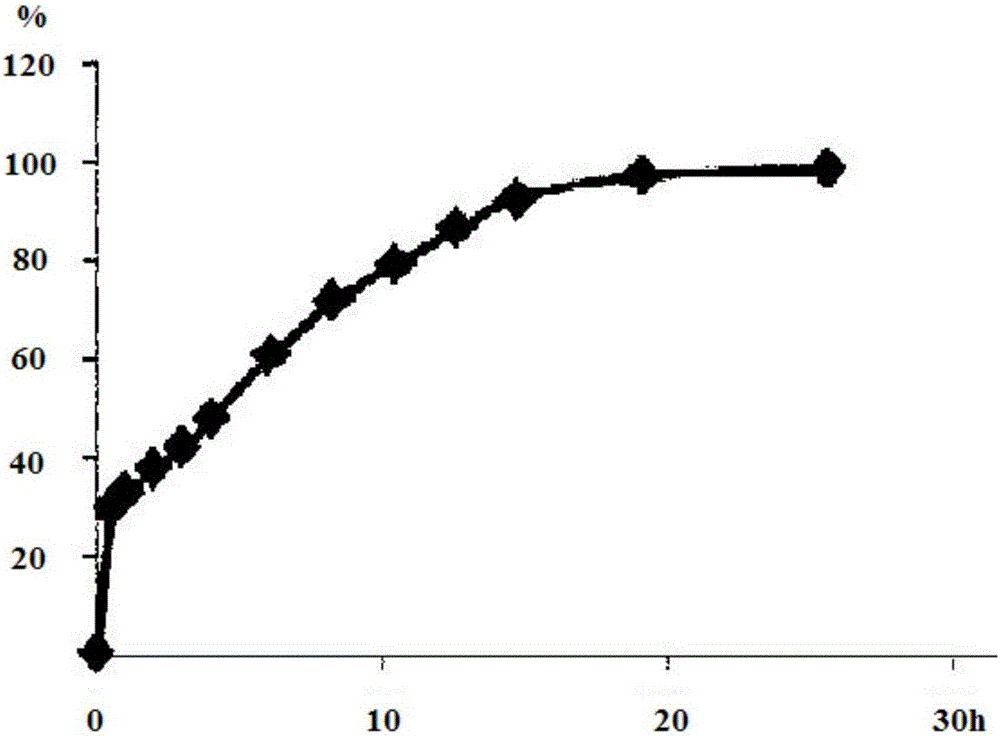
[0041] The preparation method comprises: making drug delivery systems I and II into dry granules with an appropriate amount of ethanol-water solvent with a volume ratio of 30 / 70, adding an acrylic resin copolymer with 10% by mass of drug delivery system I as an isolation layer, and then using A multi-layer tablet press is pressed into a multi-layer tablet. The tablet is coated with Opadry, and the weight gain is 3% after coating. The tablet structure refers to figure 1 . The dissolution refers to the first method for the determination of the release rate in Appendix XD of the second part of the Chinese Pharmacopoeia in 2015, and the device for the second method of the 0931 dissolution and release test method under the item of the fourth part of the Chinese Pharmacopoeia in 2015 is used. 900mL of water is the release medium, and the rotation speed is 50r / min, the temperature is 37°C, operate ...
Embodiment 2
[0044] follow the prescription
[0045] Table 2 Specifications: 200mg
[0046]
[0047]
[0048] The preparation method comprises: making drug delivery systems I and II into dry granules with an appropriate amount of ethanol-water solvent with a volume ratio of 30 / 70, adding cellulose acetate with 12% by mass of drug delivery system I as an isolation layer, and then using multiple The laminated tablet machine is pressed into a multi-layer tablet, which is coated with Opadry, and the weight gain is 3% after coating. The tablet structure refers to figure 1 shown. The release curve is basically consistent with Example 1.
Embodiment 3
[0050] follow the prescription
[0051] Table 3 Specifications: 200mg
[0052]
[0053] The preparation method comprises: making drug delivery systems I and II into dry granules with an appropriate amount of ethanol-water solvent with a volume ratio of 30 / 70, adding cellulose acetate with 10% by mass of drug delivery system I as an isolation layer, and then using multiple The laminated tablet machine is pressed into a multi-layer tablet, which is coated with Opadry, and the weight gain is 3% after coating. The tablet structure refers to figure 1 shown. The release curve is basically consistent with Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com