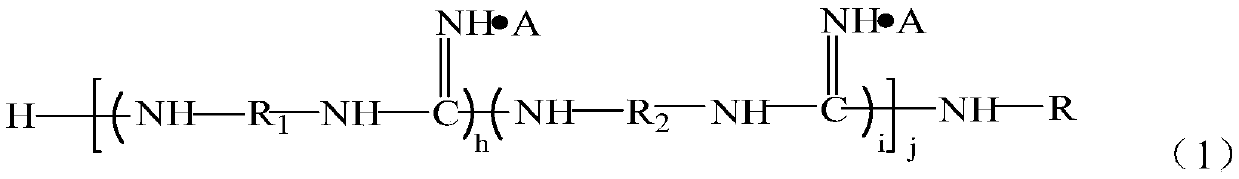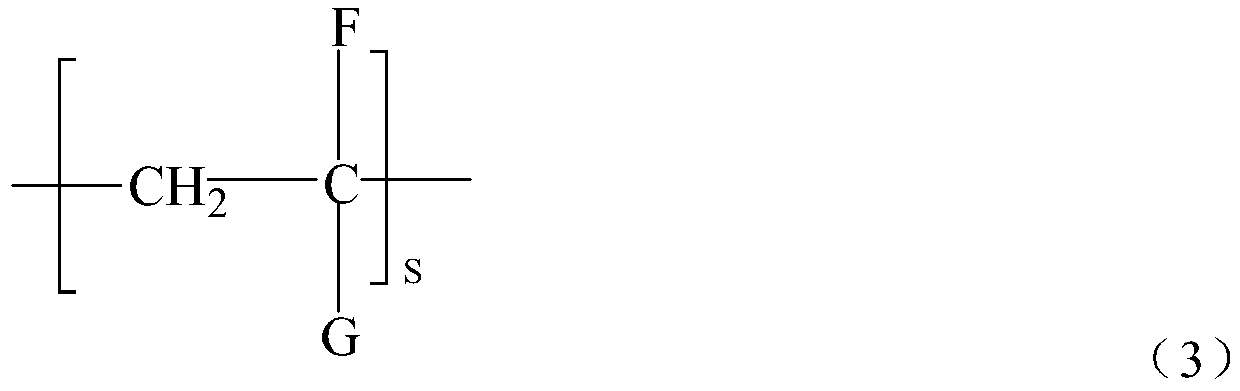Guanidine oligomer and its preparation method and its application bonded to general polymer molecular chains
A technology of oligomers and bonding, which is applied in the application field of guanidine oligomers and their preparation methods and bonding to general polymer molecular chains, and can solve problems that affect the mechanical properties of materials and cannot function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
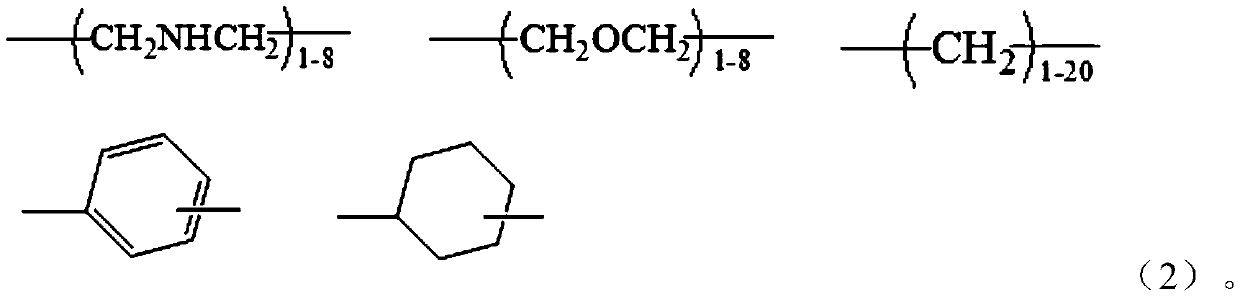
[0084] Get α, ω-tetraethylene glycol diamine 114g, 1,8-octyl diamine 94g, add respectively in two 500ml three-necked flasks, then respectively add 48g guanidine hydrochloride in two flasks respectively, under nitrogen gas Under protection, stir and heat up to 120°C, react for 2 hours, then combine the materials in the two flasks into one, and gradually heat up to 170°C to continue the reaction for 1.5 hours, and finally add 117g octadecylamine to react for 1.5 hours, Pour out while hot to obtain the guanidinium salt copolymer. After cooling down to 130°C, slowly add and dissolve in dimethylformamide (DMF), then cool to 80°C, add 30 g of maleic anhydride and react for 100 minutes, then separate the solvent to obtain the functional group shown in formula (4) The precursor of about 185g.
[0085] Take 800g of polyethylene (relative molecular mass 50000, equivalent to about 1700 for s in formula 3) and 200g of the precursor of the above functional group, add 32g of styrene into 1...
Embodiment 2
[0090] Take 70g of 1,6-hexamethylenediamine and 86g of 1,8-octanediamine and add them to two 1000ml three-necked flasks respectively, then add 48g of guanidine hydrochloride to each of the two flasks, and stir and Raise the temperature to 120°C, react for 2 hours, then combine the materials in the two flasks into one, and gradually raise the temperature to 165°C to continue the reaction for 1.5 hours, and finally add 117g of octadecylamine to react for 1.5 hours, and pour it out while hot to obtain Guanidinium Copolymer. Slowly add and dissolve in dimethylformamide (DMF) after cooling down to 130°C, then cool to 80°C, add 62g of glycidyl methacrylate, and 1.8g of B215 antioxidant, react for 100 minutes, and separate the solvent , that is to obtain the precursor of the functional group represented by formula (4), about 290g.
[0091] Take 800g of polypropylene (relative molecular mass 40000, equivalent to about 950 in formula 3) and 200g of the precursor of the above functiona...
Embodiment 3
[0096] Get α, omega-tetraethylene glycol diamine 115g, 1,4-butanediamine 53g, add respectively in two 500ml three-necked flasks, then respectively add 48g guanidine hydrochloride in two flasks respectively, under nitrogen Under protection, stir and heat up to 120°C, react for 2 hours, then combine the materials in the two flasks into one, and gradually heat up to 170°C to continue the reaction for 1.5 hours, and finally add 80g of laurylamine to react for 1.5 hours. Hot decanting to obtain guanidinium salt copolymer. After cooling down to 130°C, slowly add and dissolve in dimethylformamide (DMF), add 31g of acrylic acid, and 1.8g of B215 antioxidant, react for 200 minutes, separate the solvent, and obtain the precursor of the functional group represented by formula (4) Body, about 290g.
[0097] Take 800g of polyethylene EVA (relative molecular mass: 20000) and 200g of the precursor of the above-mentioned functional groups, add 60g of styrene into 1500mL of xylene, then add 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com