Amino functional group chiral compound resolution mark band fluorescence derivatizing agent
A chiral compound, fluorescence derivatization technology, applied in the field of biological analysis, can solve the problem of incomplete chiral resolution, and achieve the effect of reliable fluorescent chiral derivatization reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
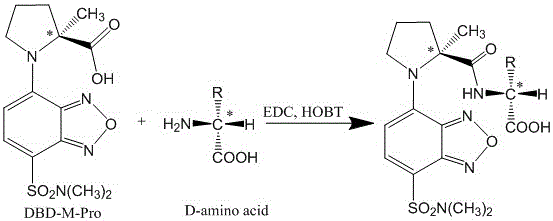
[0018] Synthesis of DBD-M-Pro:
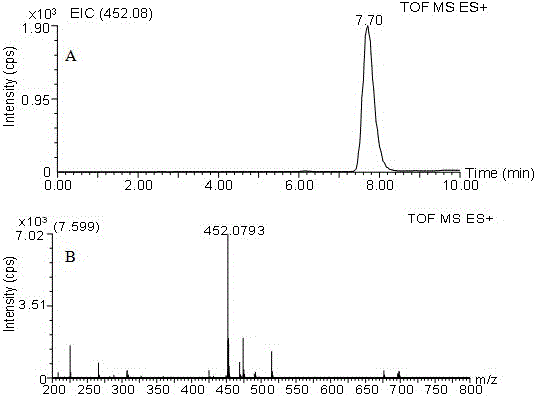
[0019] 100 mg of DBD-F is dissolved in 10 mL of acetonitrile solution, 54 mg of M-L-Pro is dissolved in 10 mL of 0.25 M Na 2 CO 3 In the solution, the two solutions were mixed and reacted on a thermostatic magnetic stirrer at 30°C for 1 hour, and the solvent was evaporated under reduced pressure. The obtained residue was redissolved in 20 mL of water, and then the same volume of ethyl acetate was added. After fully standing still, the ethyl acetate layer was discarded, and the pH of the aqueous layer was adjusted to 1-2 with 2.0 mol / L HCl. Then, the same amount of ethyl acetate was added for extraction, and the extraction was repeated three times. The combined ethyl acetate layer was dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain 78.6 mg of yellow powder. LC-ESI-TOF-MS spectral data, (m / z): 355.06 [M+H] + , t R =6.23 min; 1 H-NMR (300 MHz, CDCl 3 ) δ7.89 (t, J = 14.2 Hz, 1H), 6.00 (d, J ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com