Recombinant engineering bacterium with surface exhibiting and expressing glutamic acid decarboxylase as well as construction method and application of recombinant engineering bacterium
A technology of glutamic acid decarboxylase and recombinant engineering bacteria, which is applied in the biological field, can solve problems such as insufficient apparent catalytic activity, cumbersome steps, and the impact of mass transfer resistance, and achieve the effects of easy industrial application, simple equipment, and simplified operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 , Construction of Escherichia coli Intracellular Expression and Surface Display Glutamate Decarboxylase Recombinant Plasmid
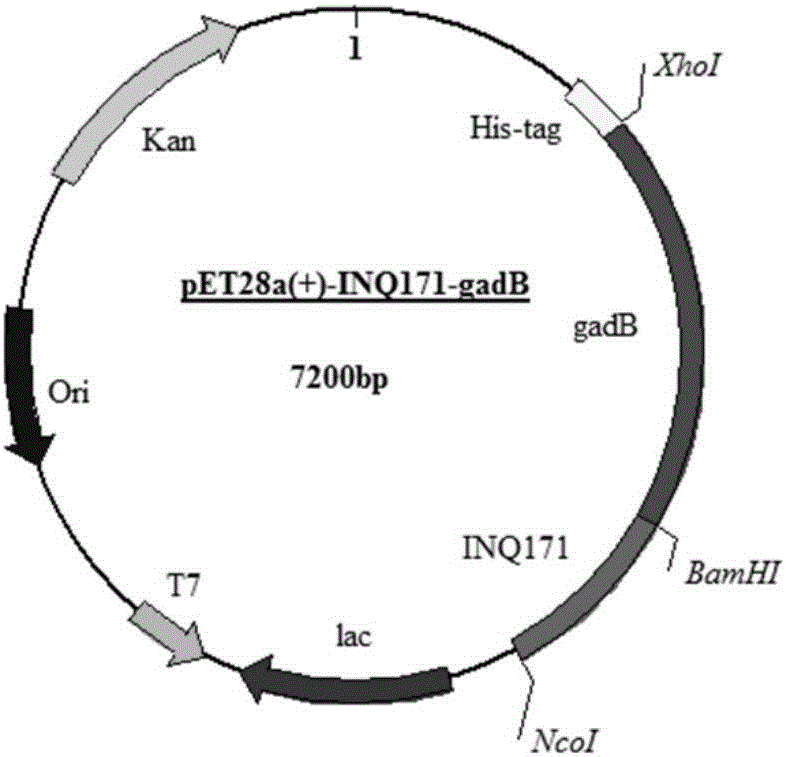
[0055] The construction process of recombinant plasmids for intracellular expression and surface display of glutamic acid decarboxylase in Escherichia coli is as follows: figure 1 shown. According to the β subtype gene sequence of the GAD of Escherichia coli in GenBank (see SEQ ID No.1 for the specific sequence, or GenBank No.EF551361.1), design and synthesize primers gadB-F and gadB-R, and its sequence is as follows:
[0056] gadB-F: 5'-AATT GGATCC ATGGATAAGAAGCAAG-3', the base corresponding to the underline indicates the BamH I restriction site;
[0057] gadB-R: 5'-AAGG CTCGAG GGTATGTTTAAAGCTG-3', the base corresponding to the underline indicates the Xho I restriction site.
[0058] Using the total DNA of Escherichia coli strains as a template, PCR amplification was performed using the primer pair gadB-F and gadB-R, and the re...
Embodiment 2
[0065] Example 2 Expression of Recombinant Intracellular GAD Enzyme and Recombinant Surface Displayed GAD Enzyme in Escherichia coli
[0066] The plasmids pET28a-gad B, pET28a-INP171-gadB and pET28a-INP224-gadB were respectively transformed into E.coli BL21(DE3) competent cells, and the plates containing LB solid medium containing 50ug / mL kanamycin were coated, 37 Incubate overnight at ℃. Single clones were picked, inoculated into LB liquid medium containing 50ug / mL kanamycin, and cultured at 37°C in a constant temperature shaker at 180rpm for 10-12h. Take the culture solution and inoculate it into fresh LB medium containing 50ug / mL kanamycin with a 2% inoculum amount, shake it in a shaker at 37°C and 180rpm until the OD600 reaches 0.6-0.8, add a final concentration of 0.4mM IPTG, 16°C, 180rpm continued shaking culture for 14-16h to induce the expression of GAD enzyme.
[0067] Collect the cells by centrifugation at 5000×g at 4°C for 20 minutes, wash once with citric acid-...
Embodiment 3
[0069] Example 3 Analysis of the apparent enzyme activity of recombinant engineering bacteria containing intracellular expression of GAD enzyme and surface display expression of GAD enzyme
[0070] Get the thalli that 10OD embodiment 2 obtains, centrifuge and collect thalline. Resuspend in 0.2M pH4.0 citric acid-disodium hydrogen phosphate buffer containing 0.1M L-sodium glutamate and 10mMPLP, react at 30°C for 15min at 180rpm / min. Centrifuge at 12000rpm for 30min, take 10ul supernatant, and use Berthelot chromogenic method to measure the amount of GABA produced.
[0071] It has been determined that the apparent enzyme activity of the recombinant engineering bacteria expressing GAD enzyme on the surface is 200U / g, while the apparent enzyme activity of the recombinant engineering bacteria expressing GAD enzyme in the cells under the same culture conditions is 170U / g, the former is 17.65% higher than the latter .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com