Bacillus subtilis cjp3 for producing CotA laccase as well as CotA laccase and application thereof
A technology of Bacillus subtilis and laccase, which is applied in the field of applied microorganisms, can solve the problems of slow expression growth cycle of fungi and easy generation of inclusion bodies, etc., and achieves the effects of strong applicability, good stability, and mature and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Cloning and expression purification of CotA laccase gene
[0037] 1. Cloning of the CotA laccase gene
[0038] The pure colony of Bacillus subtilis cjp3 (CCTCC NO: M 2016408) was picked into 5 mL LB liquid medium, cultivated overnight at 37°C and 200 rpm. Genomic DNA of the above strains was extracted by CTAB / NaCl method, and detected by 1% agarose gel electrophoresis.
[0039] Use genomic DNA as a template and upstream primer CotA-F:
[0040] 5′-ATAAGAATGCGGCCGCATGACACTTGAAAAATTTG-3′ (containing Not I restriction site) and downstream primer CotA-R: 5′-CCGCTCGAGTTATTTATGGGGATCAGTT-3′ (containing Xho I restriction site) amplified the CotA laccase gene of strain cjp3.
[0041] PCR system: ddH 2 O 22μL, 10×Fast Pfu Buffer 10μL, 10mmol / L dNTP mixture 4μL, PCR stimulate 10μL, 100mmol / L upstream and downstream primers 1μL, DNA template 1μL, 2.5U / L Fast Pfu enzyme 1μL.
[0042] The amplification program was: 98°C for 3min pre-denaturation, 95°C for 30s, 55°C for ...
Embodiment 2
[0049] The properties of embodiment 2 recombinant laccase
[0050] 1. The effect of pH on CotA laccase activity
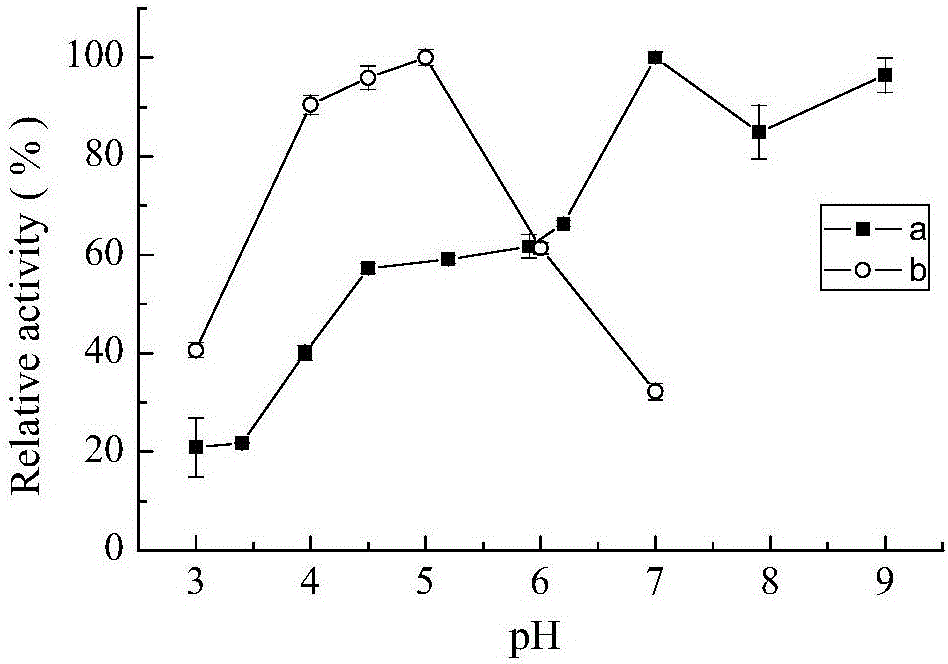
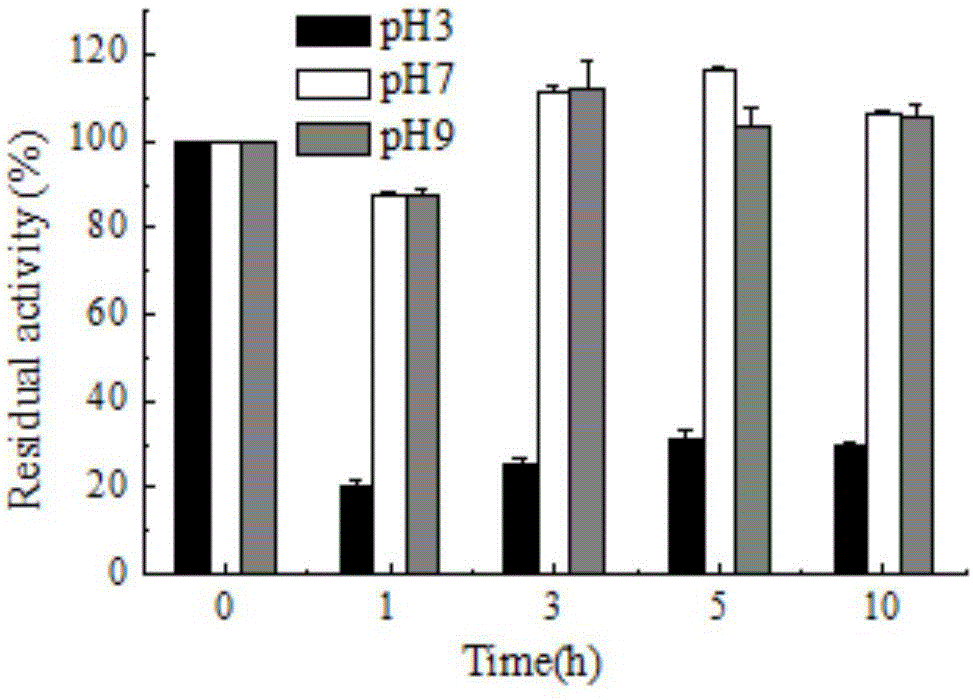
[0051] The effect of pH on laccase activity was measured by 0.1M citric acid-phosphate buffer (pH 3.0-7.0), 0.1M Tris-HCl buffer (pH7.0-9.0). The optimum pH of laccase enzyme activity was measured with ABTS in 0.1M citric acid-phosphate buffer (pH 3.0-7.0). The effect of pH on the stability of bacterial laccase was determined by measuring the remaining enzyme activity after incubation at 30°C for several hours at pH 3.0, 7.0, 9.0. The result is as figure 1 As shown, it shows that the CotA laccase provided by the present invention has a wide range of catalysis, and can catalyze substrate reactions in the range of pH 3-9. When ABTS was used as the substrate, the optimum pH measured was 5, figure 2 It shows that CotA laccase has good stability in the environment of pH9.0, and can still maintain high activity after 10h.
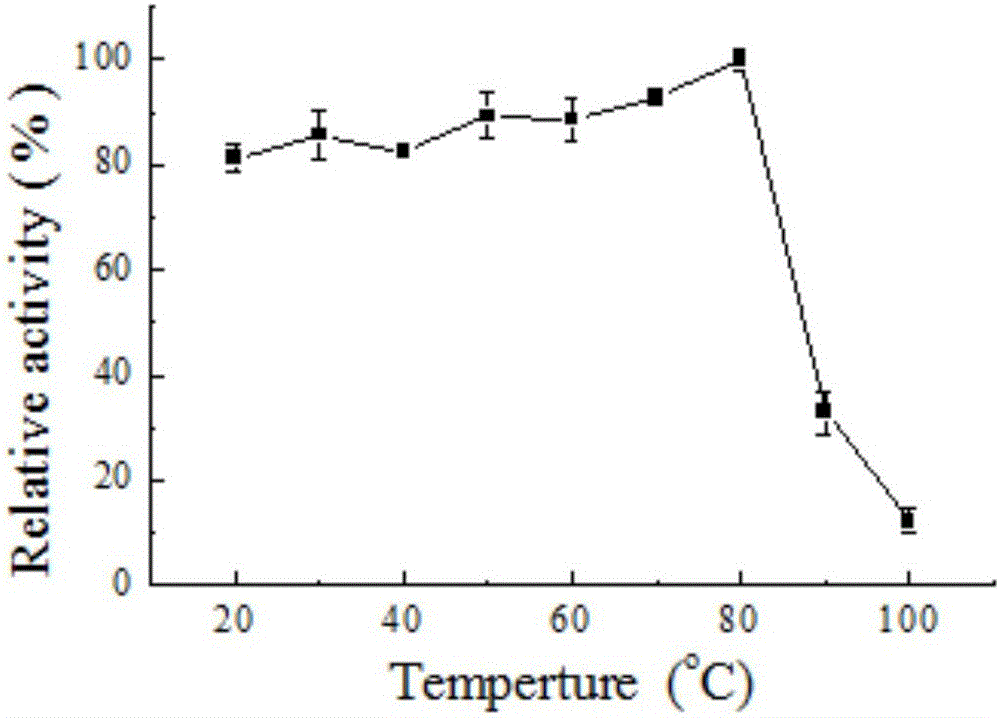
[0052] 2. The effect of temperature on th...
Embodiment 3
[0064] Embodiment 3 recombinant laccase is to the decolorization of synthetic dyestuff
[0065] 1. The reaction system of the decolorization experiment and the calculation of the decolorization rate
PUM
| Property | Measurement | Unit |
|---|---|---|
| decolorization rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com