Antibody type molecular chaperone capable of inhibiting Tau protein from being aggregated
A single-chain antibody, nucleic acid molecule technology, applied in the direction of antibodies, immunoglobulins, anti-animal/human immunoglobulins, etc., can solve the problems of the side effects of kinase inhibitors and the difficulties of kinase inhibitors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
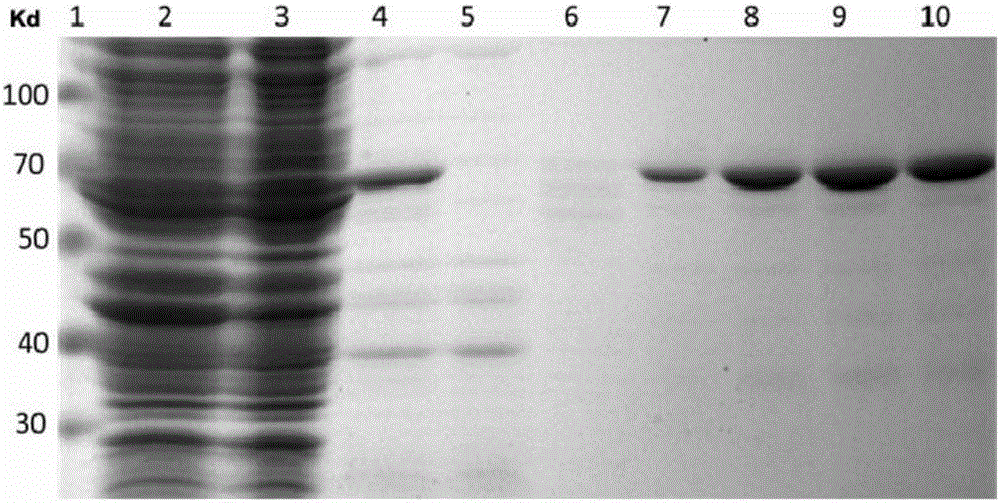
[0115] Example 1. Prokaryotic expression, separation and purification of Tau protein
[0116] 1. Prokaryotic expression, separation and purification of Tau protein
[0117] 1. Construction of pET21a-Tau plasmid
[0118] Using pGEX-6p-1-Tau plasmid as a template for PCR amplification, the PCR product, which is the Tau gene fragment, was obtained; the PCR product and pET21a plasmid were double digested with restriction enzymes NdeⅠ and XhoⅠ, respectively After the Tau gene and pET21a plasmid backbone vector; ligate the digested Tau gene and pET21a plasmid backbone vector to obtain pET21a-Tau plasmid and verify it by sequencing.
[0119] Sequencing results show that the pET21a-Tau plasmid inserts the DNA fragment shown in sequence 1 into the pET21a plasmid (purchased from Merck Millipore, catalog number 69740-3CN) between the NdeI and XhoI restriction sites, and maintains other sequences of the pET21a plasmid The resulting plasmid is unchanged.
[0120] 2. Construction of recombinant bac...
Embodiment 2
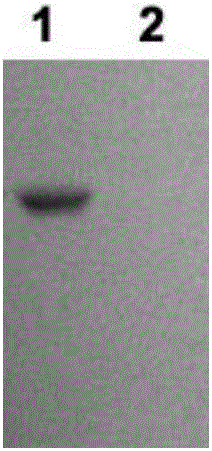
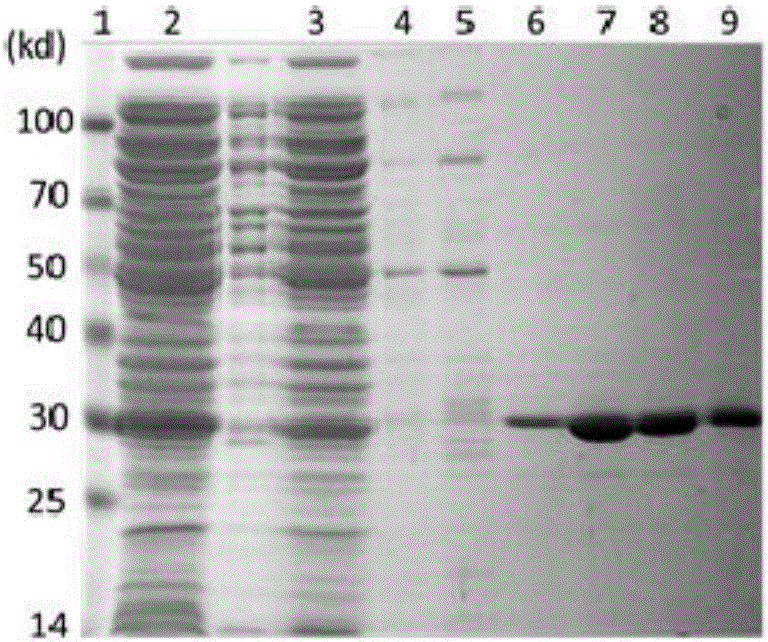
[0149] Example 2. Preparation, separation and purification of single-chain antibody
[0150] 1. Screening and identification of phage antibodies
[0151] 1. Screening of phage antibodies
[0152] Using the phosphorylated Tau protein prepared in Example 1 as the antigen, a large-capacity human phage antibody library was used to screen and obtain scFv single-chain antibodies with chaperone function and inhibit the aggregation of phosphorylated Tau protein. Specific steps are as follows:
[0153] (1) Coating: Coat the immunotube with the phosphorylated Tau protein prepared in Example 1, at 4°C overnight;
[0154] (2) Sealing: Sealing with 30g / L skimmed milk powder for 1 hour;
[0155] (3) Incubation: Wash twice with phosphate buffer containing 0.05% Tween-20, add 1 mL of phage antibody library (entrusted to prepare by the Central Laboratory of Naval General Hospital, titer is 3×10 13 CFU / mL), incubate at 37°C for 2 hours;
[0156] (4) Washing: Repeated washing to remove unbound phage antibo...
Embodiment 3
[0217] Example 3. The effect of single-chain antibody scFv on the aggregation of phosphorylated Tau protein
[0218] 1. The effect of scFv on phosphorylated Tau protein aggregation in dilute solution
[0219] 1. The effect of different scFv on the aggregation of phosphorylated Tau protein
[0220] According to the difference of single chain antibodies, they are divided into the following groups to study the effect of single chain antibodies on phosphorylated Tau protein aggregation:
[0221] Group 1 (control): Phosphorylated Tau protein (prepared in Example 1, final concentration of 5μM), heparin (purchased from Sigma-Aldrich, catalog number H3149, final concentration of 1.25μM), DTT (purchased from Sigma -Aldrich, catalog number D0632, final concentration is 1mM) and Tris-HCl buffer (10mM, pH7.5), mix well, incubate at 37℃, and use ThT fluorescence method to detect the aggregation of phosphorylated Tau protein at different times;
[0222] Group 2 (scFv T1): mix scFv T1 (purified sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com