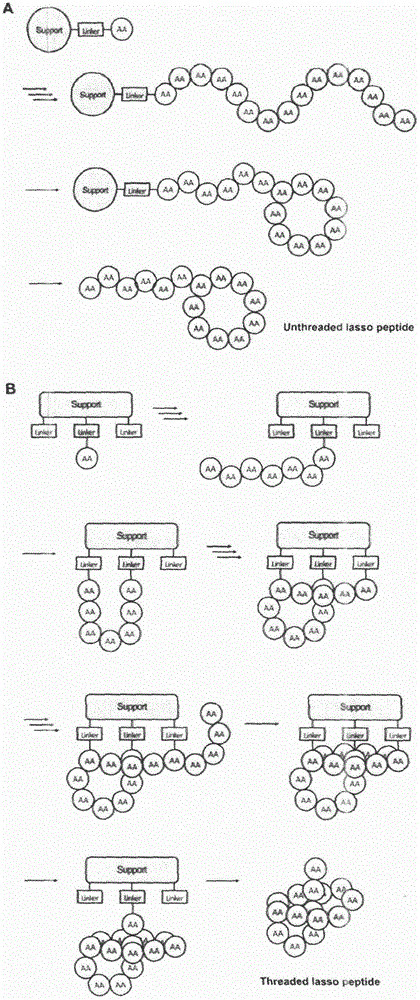
Complete chemical synthesis for lasso polypeptide
A lasso and chemical technology, applied in the field of synthesis of lasso polypeptides and their analogs, can solve the problems of rapid increase in marketization cost and difficulty of popularization, unfavorable drug research and development screening and modification, poor controllability of lasso polypeptide synthesis, etc. Achieve the effect of being conducive to automation and large-scale synthesis, easy to control, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
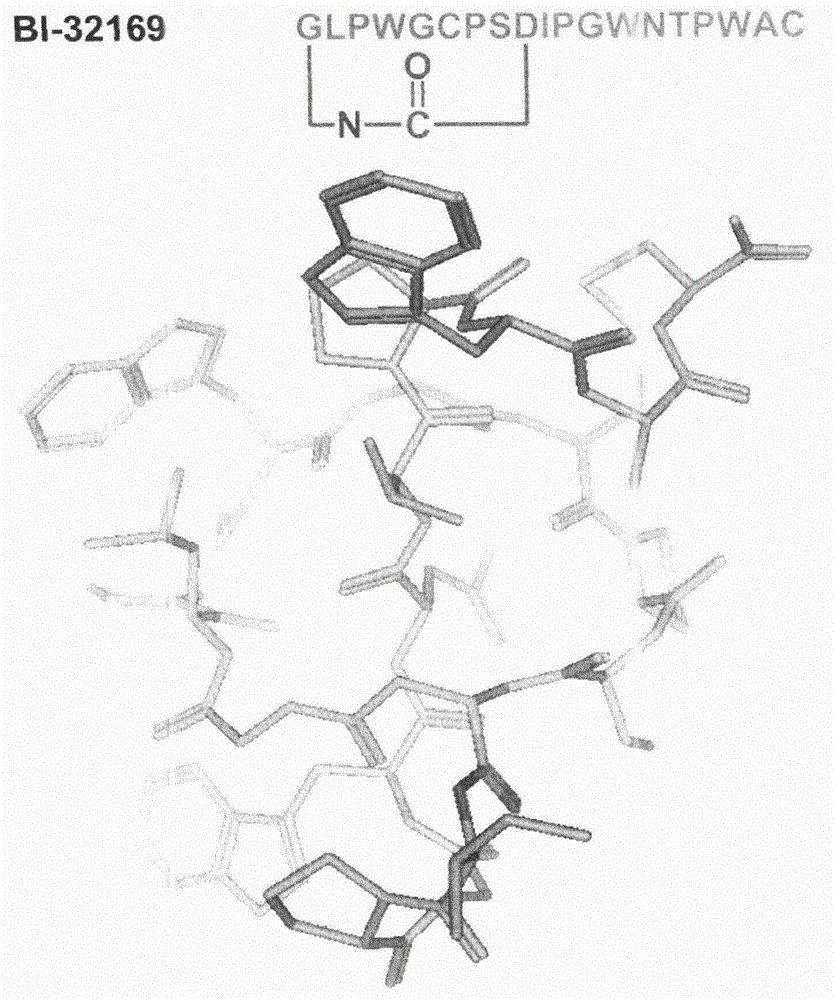
[0035] The target product is the natural lasso polypeptide BI-32169 with glucagon receptor antagonist, the sequence and the spatial configuration of the computer simulation are as follows figure 2 shown. Wherein, G in the sequence line is glycine, L is leucine, P is proline, W is tryptophan, C is cysteine, S is serine, D is aspartic acid, and I is isoleucine Amino acid, N is asparagine, T is threonine, A is alanine.
[0036] Step 1, the loading of the first amino acid in the ionic liquid. The imidazole ionic liquid 1,3-dimethyl-2-hydroxymethylimidazolium tetraphenylborate is used as the initial support, and the structural formula is: 1.0 equivalent (110.0 μL) of ionic liquid was dissolved in 1.2 mL of dichloromethane, and 2.0 equivalent of fluorenylmethoxycarbonyl-protected cysteine was added. After stirring and dissolving, 4.0 equivalents of condensing agent N,N'-dicyclohexylcarbodiimide and 1.0 equivalents of catalyst 4-dimethylaminopyridine were added. After stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com