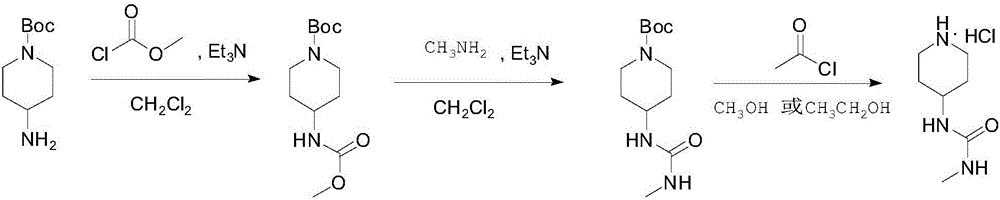
Synthesis method of 1-methyl-3-(piperidine-4-yl) urea hydrochloride
A synthesis method and technology of hydrochloride, applied in organic chemistry and other directions, can solve problems such as easy moisture absorption, and achieve the effects of easy operation, mild reaction conditions and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Dissolve 2.0kg (about 10mol) of N-Boc-4-aminopiperidine, 1.0kg (about 11mol) of methyl chloroformate and 3.1kg (about 31mol) of triethylamine in a suitable reactor into 20L of dichloromethane (the molar ratio of N-Boc-4-aminopiperidine, methyl chloroformate, and triethylamine is about 1:1.1:3.1), and stirred at 25°C for 2 hours. TLC tracking showed that the starting material disappeared. 10 L of water was added to the reaction solution, the organic layer was separated, and the aqueous phase was extracted with dichloromethane (2×5 L). The organic phases were combined, washed successively with water (4 L) and saturated brine (4 L), dried with 2 kg of anhydrous sodium sulfate, filtered, and concentrated to obtain 2.64 kg of N-Boc-4-[(methoxyacyl)amino ]piperidine (LCMS[M+Na] + 281.1), with a purity of about 95%, about 9.7 mol. This product was used directly in the next reaction.
[0032] (2) In a suitable reactor, mix 2.64kg (about 9.7mol) of N-Boc-4-[(methoxyacyl)a...
Embodiment 2
[0037] (1) Dissolve 20.0kg (about 100mol) of N-Boc-4-aminopiperidine, 12.3kg (about 130mol) of methyl chloroformate and 33.4kg (about 330mol) of triethylamine in a suitable reactor into 100L of dichloromethane (the molar ratio of N-Boc-4-aminopiperidine, methyl chloroformate and triethylamine is about 1:1.3:3.3), the mixture was stirred at 20°C for 3 hours, TLC Trace shows ingredients disappearing. 100L of water was added to the reaction solution, the organic layer was separated, and the aqueous phase was extracted with dichloromethane (2×50L). The organic phases were combined, washed with water (40L) and saturated brine (40L) successively, dried with 20kg of anhydrous sodium sulfate, filtered, and concentrated to obtain 25.83kg of N-Boc-4-[(methoxyacyl)amino]piperene Pyridine (LCMS[M+Na] + 281.1), the purity is about 94%, about 94mol. This product was used directly in the next reaction.
[0038] (2) 25.83kg (about 94mol) of N-Boc-4-[(methoxyacyl)amino]piperidine and 61.1L...
Embodiment 3
[0043] (1) Dissolve 200.0kg (1000mol) of N-Boc-4-aminopiperidine, 141.8kg (1500mol) of methyl chloroformate and 303.6kg (3000mol) of triethylamine into 1200L diethylamine in a suitable reactor In methyl chloride (the molar ratio of N-Boc-4-aminopiperidine, methyl chloroformate, and triethylamine is about 1:1.5:3.5), stir at 25°C for 3 hours, TLC tracking shows that the raw materials disappear, and the reaction is complete 1000L of water was added to the final reaction solution, the organic layer was separated, and the aqueous phase was extracted with dichloromethane (2×500L). Combine the organic phases, wash with water (500L) and saturated brine (400L) successively, then dry with 250kg of anhydrous sodium sulfate, filter, and concentrate to obtain 250.23kg of N-Boc-4-[(methoxyacyl)amino] Piperidine (LCMS[M+Na] + 281.1), the purity is about 96%, about 930mol. This product was used directly in the next reaction.
[0044] (2) In a suitable reactor, combine about 250.23kg (abou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com