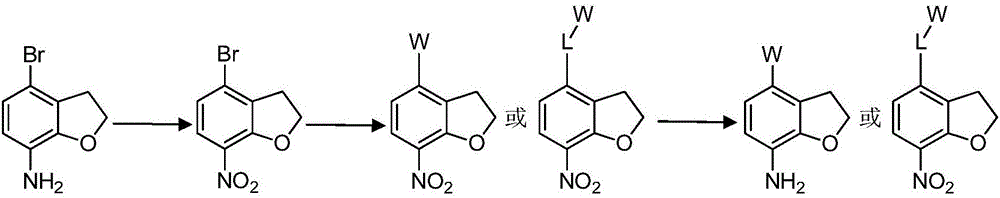
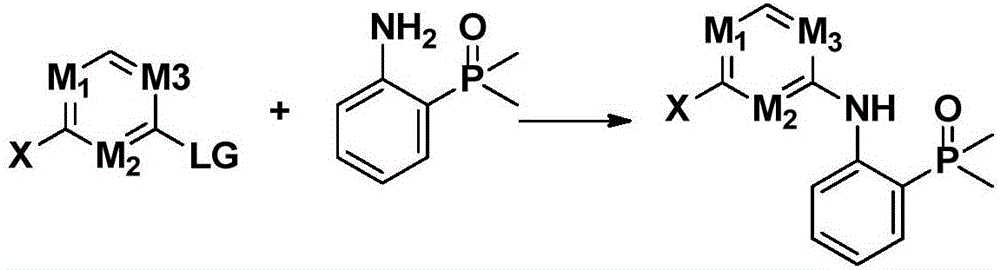
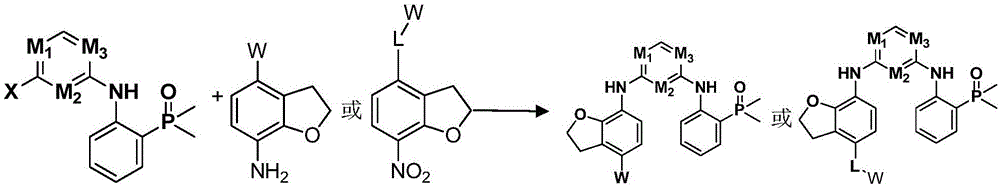
Nitrogen-containing heterogeneous ring compound, preparation method thereof and application of nitrogen-containing heterogeneous ring compound in inhibition of kinase activity
A compound and solvate technology, applied in the field of medicinal chemistry, can solve problems such as low selectivity and poor curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0169] Intermediate 11 (25mg, 0.10mmol) and Intermediate 20 (35mg, 0.12mmol) were dissolved in iPrOH (1mL), concentrated hydrochloric acid (24mg, 0.25mmol) was added, microwave reaction (250w, 130°C, 120min, 300psi) . Cool to room temperature, add EA (20mL), wash with saturated sodium bicarbonate (aq) (20mL×3), wash with saturated brine (20mL×3), column chromatography (D / M / Et 3 N=10:1:0.02), to obtain Example 1: 48 mg of off-white solid with a yield of 71%.
[0170] The structure verification data is as follows: 1 H NMR (CDCl 3 )δ (ppm): 10.01 (s, 1H), 8.46 (dd, J 1 =8.4Hz,J 2 =4.4Hz,1H),8.35(s,1H),7.47(t,J=7.6Hz,1H),7.23-7.15(m,2H),7.02-6.98(m,1H),6.45(d,J= 8.8Hz, 1H), 6.35(s, 1H), 5.87(s, 1H), 4.60(t, J=8.8Hz, 2H), 3.40(d, J=12.0Hz, 2H), 3.20(t, J= 8.8Hz, 2H), 2.67(t, J=12.0Hz, 2H), 2.33(s, 6H), 2.29-2.23(m, 1H), 1.93(d, J=12.0Hz, 2H), 1.80(s, 3H),1.74(s,3H),1.68-1.58(m,2H).HRMS(ESI):m / z,calcd for C 27 h 36 N 6 o 2 P[M+H + ]:507.2637, found 507.2646.
Embodiment 2
[0172]Using Intermediate 11 (50 mg, 0.19 mmol) and Intermediate 21 (71 mg, 0.24 mmol) as raw materials, the same method as Example 1 was used to synthesize Example 2: 84 mg of off-white solid with a yield of 84%.
[0173] The structure verification data is as follows: 1 H NMR (DMSO-d 6 )δ (ppm): 11.51 (s, 1H), 8.66 (m, 1H), 8.24 (s, 1H), 8.02 (d, J = 3.2Hz, 1H), 7.54 (dd, J 1 =13.6Hz,J 2 =7.6Hz, 1H), 7.32(t, J=7.6Hz, 1H), 7.21(d, J=8.4Hz, 1H), 7.08(t, J=7.2Hz, 1H), 6.44(d, J=8.8 Hz,1H),4.49(t,J=8.4Hz,2H),3.41-3.39(m,2H),3.16(t,J=8.0Hz,2H),3.01(m,1H),2.64(t,J =12.0Hz,2H),2.53(s,6H),2.01(d,J=10.0Hz,2H),1.80(s,3H),1.77(s,3H),1.80-1.65(m,2H).HRMS (ESI): m / z, calcd for C 27 h 35 N 6 o 2 PF[M+H + ]:525.2543, found 525.2557.
Embodiment 3
[0175] Using Intermediate 11 (40 mg, 0.15 mmol) and Intermediate 22 (54 mg, 0.19 mmol) as raw materials, the same method as in Example 1 was used to synthesize Example 3: 35 mg of an off-white solid with a yield of 45%.
[0176] The structure verification data is as follows: 1 H NMR (DMSO-d 6 )δ(ppm):10.95(s,1H),8.51-8.50(m,1H),8.11(s,1H),7.95(d,J=5.6Hz,1H),7.52(d,J 1 =12.8Hz,J 2 =6.4Hz, 1H), 7.31(t, J=7.6Hz, 1H), 7.25(d, J=8.4Hz, 1H), 7.04(d, J=7.6Hz, 1H), 6.43(d, J=8.8 Hz,1H),5.96(d,J=5.6Hz,1H),4.49(t,J=8.4Hz,2H),3.39(m,2H),3.17-3.13(m,3H),2.63(t,J =12.0Hz,2H),2.43(s,6H),1.97-1.94(m,2H),1.78(s,3H),1.75(s,3H),1.62-1.59(m,2H).HRMS(ESI) :m / z, calcd for C 27 h 36 N 6 o 2 P[M+H + ]:507.2637, found 507.2634.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com