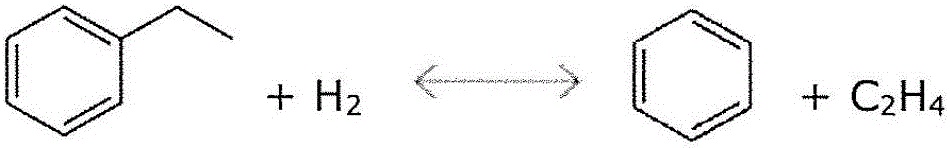Process for producing benzene from a c5-c12 hydrocarbon mixture
A C5-C12, logistics technology, applied in the direction of metal/metal oxide/metal hydroxide catalysts, hydrocarbons, hydrocarbons, etc., can solve the problem of limited ratio control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
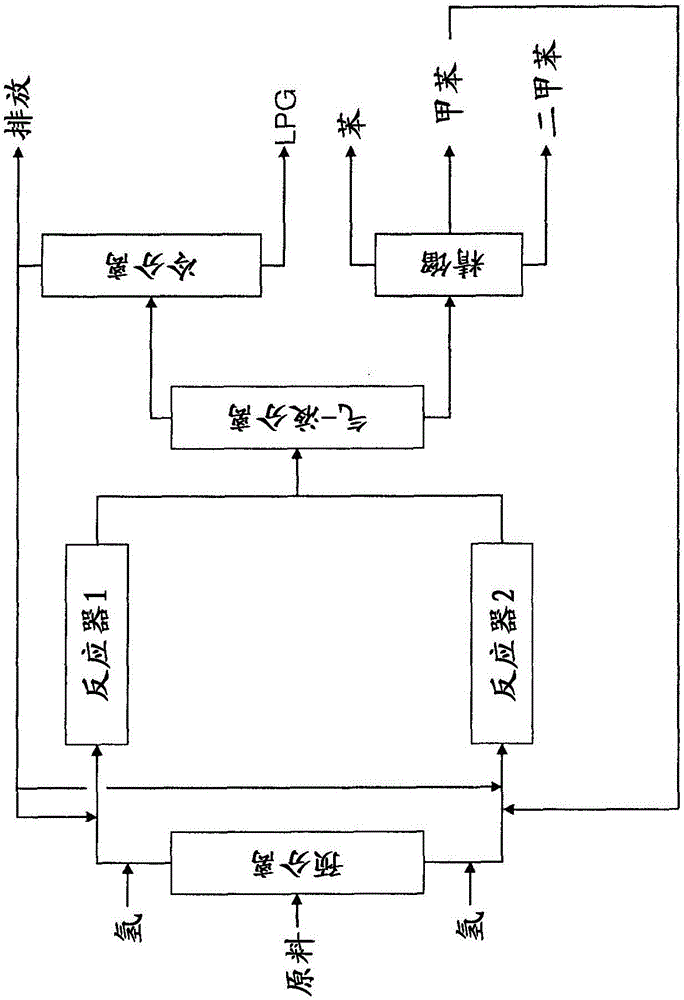
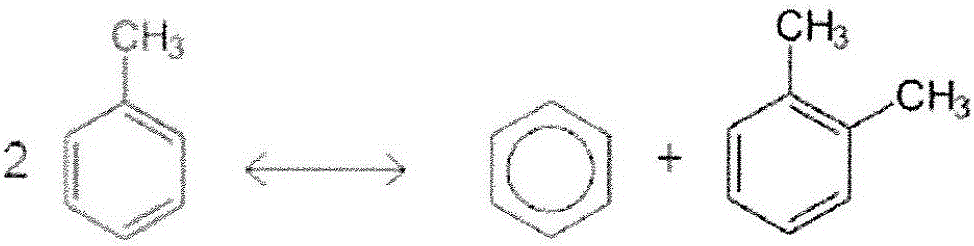
Image
Examples
Embodiment 1
[0122] Two-stage hydrotreated pyrolysis gasoline having the composition shown in Table 1 was fed to a reactor containing a hydrocracking catalyst deposited on ZSM-5 with 0.03 wt% Pt, wherein the ZSM-5 The SiO2 / Al2O3 ratio was 80. Process conditions are 476°C, 200psig, H2 / HC=3, WHSV=2hr -1 . The composition of the resulting product stream is given in Table 1 below.
[0123] The benzene in the product stream is extremely pure, ie, substantially free of benzene azeotropes.
[0124] However, the amount of benzene in the product stream is no greater than in the source feed stream. Large quantities of alkylbenzenes, especially toluene and xylenes, are obtained.
[0125] Table 1
[0126] Raw materials (wt%) Product (wt%) benzene 48.11 47.75 Cyclohexane 2.87 0.01 toluene 16.03 19.22 mixed xylenes 2.83 2.85 Ethylbenzene 5.61 0.11 trimethylbenzene 0.03 0.03 Benzene purity 71.14% 99.96%
[0127] In Table 1-4, the pu...
Embodiment 2
[0128] Embodiment 2: benzene-rich stream
[0129] A feedstock having the composition shown in Table 2 was fed into a reactor containing a hydrocracking catalyst having the same composition as the catalyst used in Example 1. The process conditions are 470°C, 200psig, H2 / HC=1, WHSV=3hr -1 . The composition of the resulting product stream is given in Table 2 below.
[0130] This example demonstrates that hydrocracking a source feedstream containing significant amounts of benzene will result in some loss of benzene, but yield reagent grade BTX from which a significant amount of reagent grade benzene can be obtained.
[0131] Table 2
[0132] Raw materials (wt%) Product (wt%) benzene 75.36 71.57 Cyclohexane 0 0.01 toluene 0 3.13 mixed xylenes 0 0.18 Ethylbenzene 0 0.25 trimethylbenzene 0 0.01 Benzene purity 78.91% 99.83%
Embodiment 3
[0133] Embodiment 3: lean benzene stream
[0134] A feedstock having the composition shown in Table 3 was fed into a reactor containing a hydrocracking catalyst having the same composition as the catalyst used in Example 1. The process conditions are 450°C, 200psig, H2 / HC=1, WHSV=3hr -1 . The composition of the resulting product stream is given in Table 3 below.
[0135] This example describes the effect of hydrocracking a low benzene feedstock stream. It can be seen that a large amount of toluene is converted to benzene and toluene by toluene disproportionation.
[0136] table 3
[0137] Raw materials (wt%) Product (wt%) benzene 0 8.88 Cyclohexane 0 0 toluene 100 78.25 mixed xylenes 0 11.26 Ethylbenzene 0 0.01 trimethylbenzene 0 0.05 Benzene purity - 99.96%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com