A kind of spin cross-fluorescence bifunctional complex, preparation method and application thereof
A technology of spin crossing and complexes, which is applied to iron group organic compounds without C-metal bonds, iron organic compounds, organic chemical methods, etc., can solve the problem that the single crystal structure of spin crossing units is not determined and cannot be well formed. Determine the relationship between structure and performance, etc., to achieve non-contact detection, to facilitate the study of the relationship between structure and performance, and to clarify the relationship between structure and performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
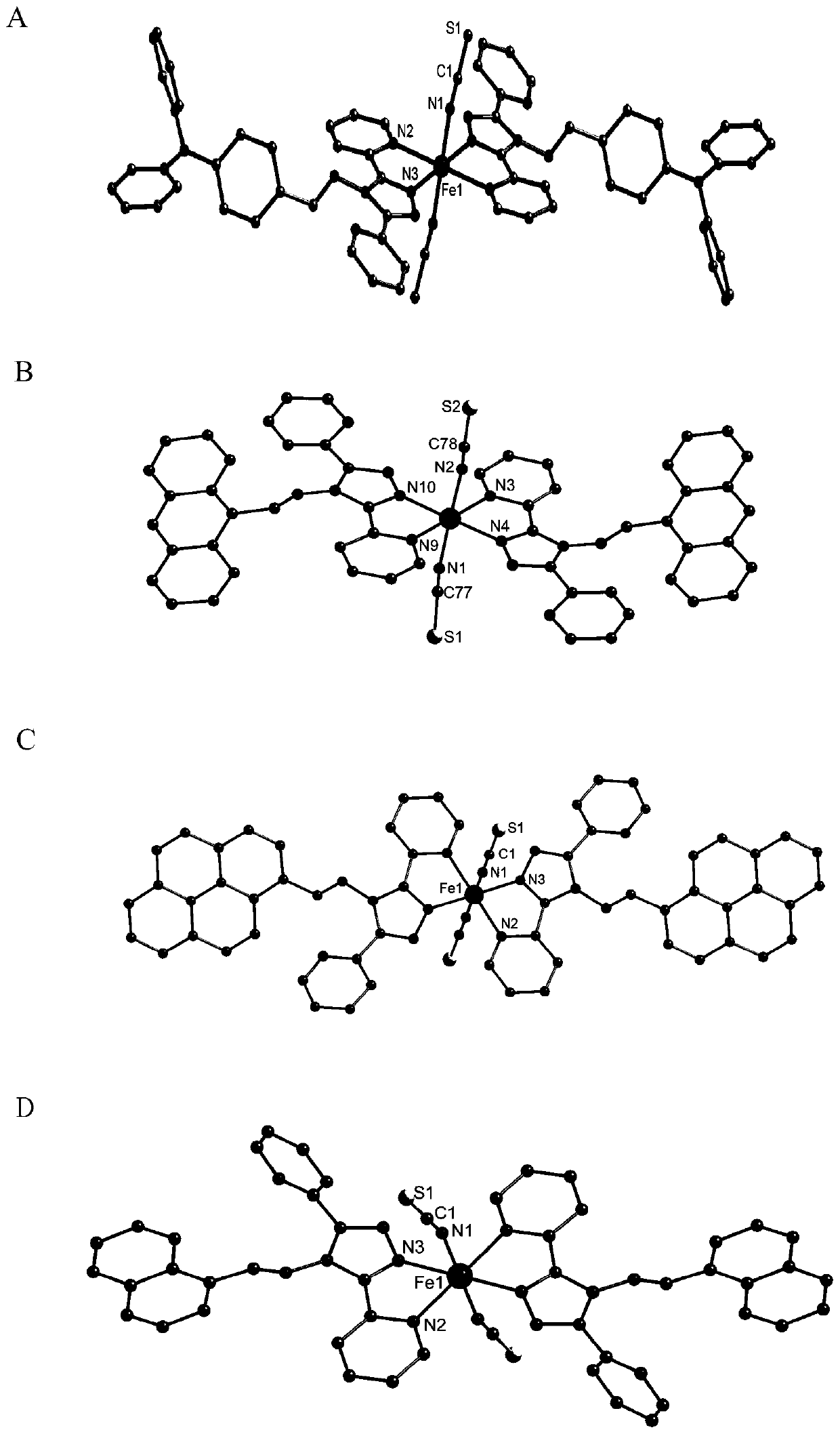
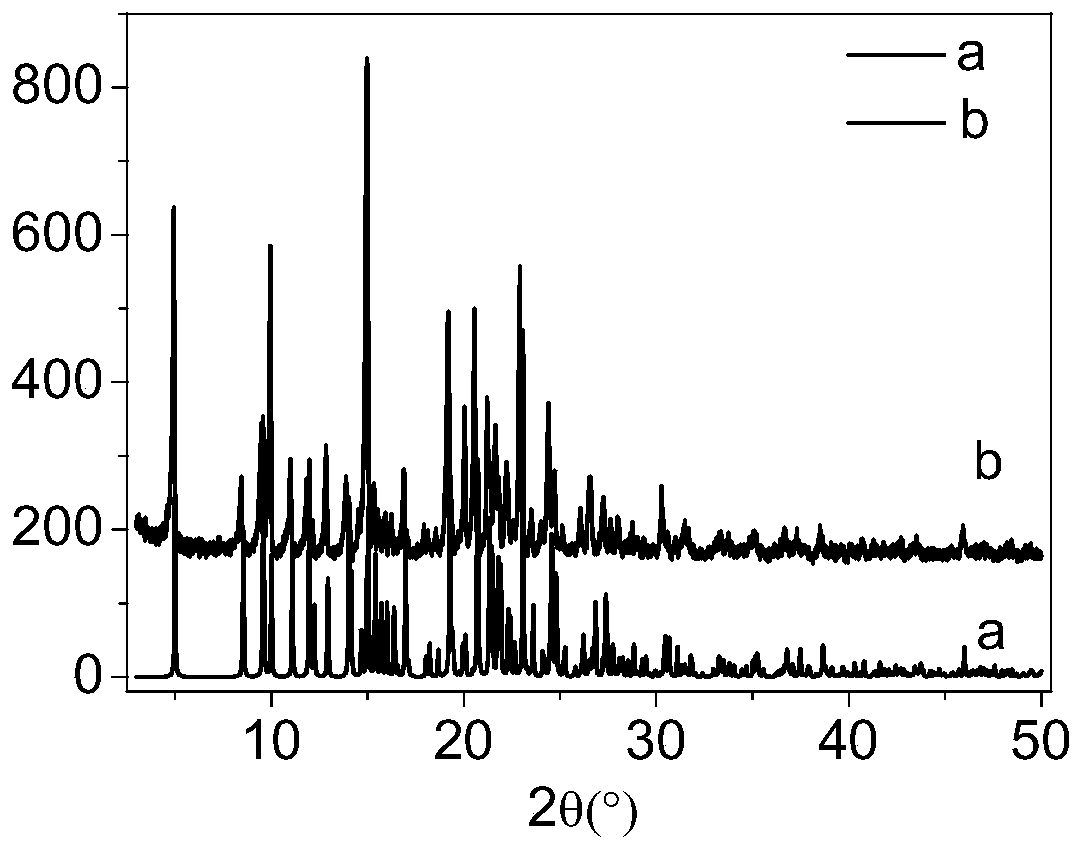
Image
Examples
Embodiment 1
[0037] (1) Preparation of L1:
[0038] Add 1.20 mmol of triphenylamine monoformaldehyde and 1.00 mmol of 3,5-dipyridine-4-amino-1,2,4-triazole into a 100 ml three-necked round-bottomed flask containing 20 ml of methanol. Under magnetic stirring, slowly heat to 85°C, and when all raw materials are completely dissolved, slowly add 3 drops of glacial acetic acid dropwise. After stirring at 85°C for 6h, the reaction was stopped. The resulting reaction solution was cooled to room temperature, and a bright yellow precipitate precipitated out, filtered, washed with methanol three times, and dried to obtain L1 with a yield of 65%. MS (ES-API) C 31 h 23 N 7 m / z:494.2[M+H] +. 1 H NMR (400MHz, CD 3 CN) δ8.58(d, J=4.8Hz, 2H), 8.56(s, 1H), 8.11(d, J=7.9Hz, 2H), 7.92(td, J=7.8, 1.8Hz, 2H), 7.58 (d, J = 8.8Hz, 2H), 7.44–7.36 (m, 6H), 7.22–7.17 (m, 6H), 6.93 (d, J = 8.8Hz, 2H).
[0039]
[0040] (2) Fe(SO 4 ) 2 4H 2 An aqueous solution of O (0.10 mmol) was added to a methanol s...
Embodiment 2
[0043] (1) Compound L1 was prepared according to the method of Example 1;
[0044] (2) Fe(SO 4 ) 2 4H 2 An aqueous solution of O (0.10 mmol) was added to a methanol solution of KSeCN (0.20 mmol) in the presence of ascorbic acid, and Fe(NCSe) was obtained by filtration. 2 solution, the prepared Fe(NCSe) 2 The solution was slowly added to the DMF solution of L1 (0.20 mmol), stirred at room temperature for 2 h, and the reaction was completed to obtain a clear reaction solution;
[0045] (3) The obtained clear reaction liquid was placed in diethyl ether vapor, after 17 days, red blocky crystals were precipitated, filtered and dried to obtain complex 2 with a yield of about 55%.
Embodiment 3
[0047] (1) Preparation of L2:
[0048] Add 1.20 mmol of 9-anthracenecarbaldehyde and 1.00 mmol of 3,5-dipyridine-4-amino-1,2,4-triazole into a 100 ml three-necked round bottom flask containing 20 ml of methanol. Under magnetic stirring, slowly heat to 85°C, and when all raw materials are completely dissolved, slowly add 3 drops of glacial acetic acid dropwise. After stirring at 85°C for 6h, the reaction was stopped. The resulting reaction solution was cooled to room temperature, and a yellow precipitate precipitated out. It was filtered, washed with methanol three times, and dried to obtain L2 with a yield of 54%. MS (ES-API) C 27 h 18 N 6 m / z:427.2[M+H] + .
[0049]
[0050] (2) Fe(SO 4 ) 2 4H 2 An aqueous solution of O (0.10 mmol) was added to a methanol solution of KSCN (0.20 mmol) in the presence of ascorbic acid, and filtered to obtain Fe(NCS) 2 solution, the prepared Fe(NCS) 2 The solution was slowly added to the DMF solution of L2 (0.20 mmol), stirred at r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com