Preparation method for chlorbipram PDE4-inhibitor
A PDE4 and inhibitor technology, applied in the field of preparation of PDE4 inhibitors, can solve the problems of many side reactions, unstable bromide, low docking reaction yield, etc., and achieves mild reaction conditions, low equipment requirements, and easily available raw materials. cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
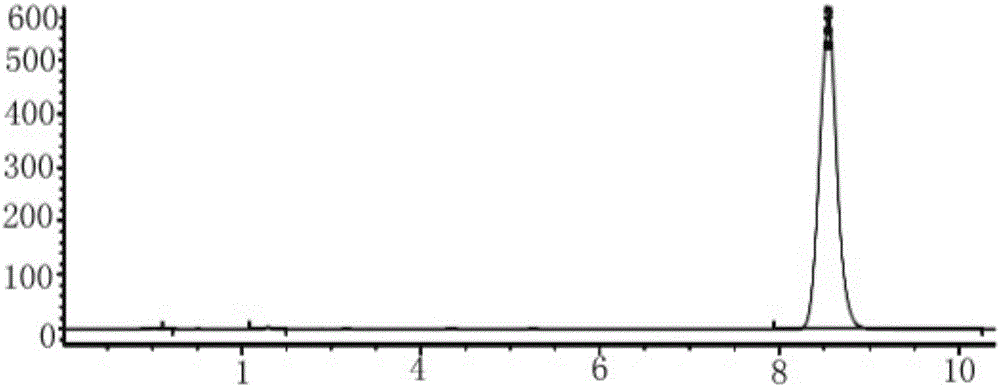
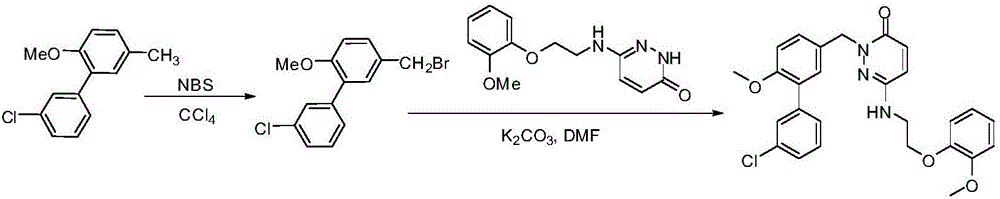
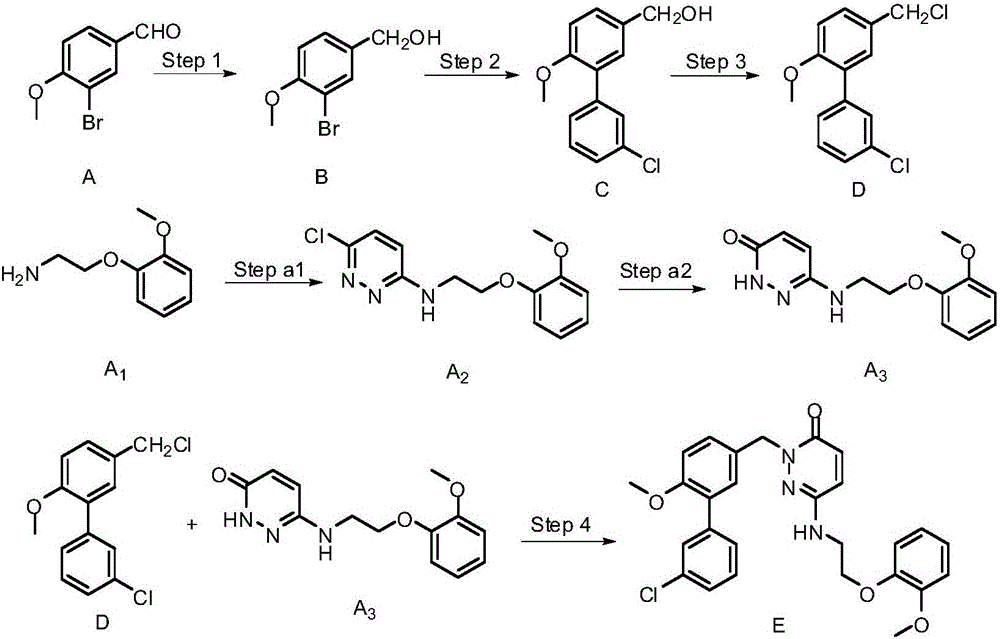
Image
Examples
Embodiment 1
[0025] (1) Take 30g of raw material A, 3-bromo-4-methoxybenzaldehyde, slowly add NaBH in batches 4 8-10g, react in ethanol. The reaction was monitored by TLC. After the reaction was complete, ethanol was removed, extracted, and the organic phases were combined, dried over anhydrous sodium sulfate, and spin-dried to obtain 30.0 g of 3-bromo-4-methoxybenzyl alcohol as a white solid. The product could be subjected to the next reaction without purification. rate>98%.
[0026] (2) Take 10 g of 3-bromo-4-methoxybenzyl alcohol, add 8.6 g of m-chlorophenylboronic acid, 18.8 g of potassium carbonate, 1.0 g of tetrakistriphenylphosphine palladium, and 100 mL of isopropanol / water solvent, and stir at room temperature. After the solid is completely dissolved, remove the oxygen in the reaction system, react without oxygen, heat, after the reaction, spin off the isopropanol and part of the water under reduced pressure, then extract with ethyl acetate, combine the organic layers, and dry ...
Embodiment 2
[0031] On the basis of Example 1, other steps remain unchanged, and the method of step (2) in Example 1 becomes the following scheme: take 10g of compound B, 3-bromo-4-methoxybenzyl alcohol, add m-chlorophenylboronic acid 8.6 g, potassium carbonate 18.8g, urea 82.8mg, palladium acetate 210mg, isopropanol / water solvent 100mL, stirred at room temperature. After the solid is completely dissolved, remove the oxygen in the reaction system, react without oxygen, heat, after the reaction, spin off the isopropanol and part of the water under reduced pressure, then extract with ethyl acetate, combine the organic layers, and dry with anhydrous sodium sulfate The palladium salt was removed by suction filtration and spin-dried to obtain Compound C, 11.46 g of light yellow viscous liquid, with a yield of >95%. The total yield of this embodiment is 65.5%.
Embodiment 3
[0033] On the basis of Example 1, other steps remain unchanged, and the method of step (3) in Example 1 becomes the following scheme: 8.13g cyanuric chloride, add DMF with slow stirring, stir at room temperature for about half an hour, add dry dichloro Methane, stir evenly, add 11.5 g of raw material C, and stir at room temperature for 4-8 hours. After the reaction, the organic layer was washed with saturated NaCl, dried and spin-dried under reduced pressure. The crude product was purified by column separation to obtain a colorless oily chlorinated product, namely compound D, 11.6 g (95% yield). The calculated total yield was 71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com