Method for preparing barium chloride and co-producing calcium sulfate whiskers by virtue of acid hydrolysis of witherite
A technology of calcium sulfate whiskers and witheraric acid, applied in chemical instruments and methods, calcium/strontium/barium chloride, single crystal growth, etc., can solve the problem that the secondary component calcium is not used reasonably, and achieve the realization of Comprehensive utilization, emission reduction and recycling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
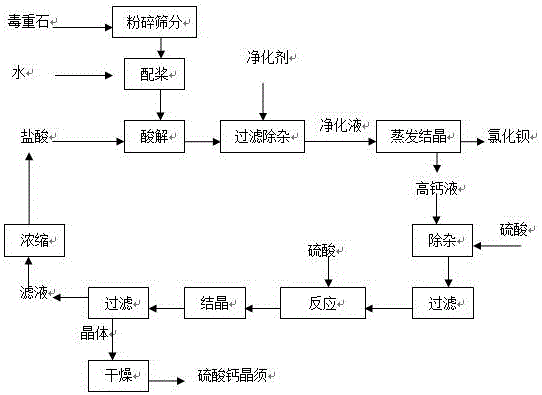
[0019] process such as figure 1 As shown, medium-grade witherite was used, and BaCO 3 Content is 55 wt %, CaCO 3 The content is 18 wt %. After crushing and sieving, take 80-120 mesh mineral powder and water to make a slurry with a solid content of 50 wt %, and then carry out acidolysis reaction with 10 wt % hydrochloric acid, and add lime after the reaction is complete Adjust the pH of the solution to 9 to remove impurities, and then filter to obtain a filter cake and filtrate. The filter cake is mainly acid-insoluble and impurities, and the filtrate is a purification solution containing calcium chloride and barium chloride. At this time, the barium chloride in the purification solution is The concentration of calcium chloride is 220 g / L, and the concentration of calcium chloride is 52 g / L.
[0020] The purification solution is concentrated and crystallized by evaporation, barium chloride crystals are separated out, and barium chloride crystals and high-calcium liquid are ob...
Embodiment 2
[0023] process such as figure 1 As shown, medium-grade witherite was used, and BaCO 3 Content is 55 wt %, CaCO 3 The content is 16 wt %. After crushing and sieving, take 80-120 mesh mineral powder and water to make a slurry with a solid content of 45 wt %, then carry out acid hydrolysis reaction with 25 wt % hydrochloric acid, and add lime after the reaction is complete Adjust the pH of the solution to 9 to remove impurities, and then filter to obtain a filter cake and filtrate. The filter cake is mainly acid-insoluble and impurities, and the filtrate is a purification solution containing calcium chloride and barium chloride. At this time, the barium chloride in the purification solution is The concentration of calcium chloride is 210 / L, and the concentration of calcium chloride is 56g / L;
[0024] The purification solution is concentrated and crystallized by evaporation, barium chloride crystals are separated out, and barium chloride crystals and high-calcium liquid are obta...
Embodiment 3
[0027] process such as figure 1 As shown, medium-grade witherite was used, and BaCO 3 Content is 50 wt %, CaCO 3 The content is 23 wt %. After crushing and sieving, take 80-120 mesh mineral powder and water to make a slurry with a solid content of 50 wt %, and then carry out acidolysis reaction with 20 wt % hydrochloric acid, and add lime after the reaction is complete Adjust the pH of the solution to 10 to remove impurities, and then filter to obtain filter cake and filtrate. The filter cake is mainly acid-insoluble and impurities, and the filtrate is a purification solution containing calcium chloride and barium chloride. At this time, barium chloride in the purification solution The concentration of calcium chloride is 180 / L, and the concentration of calcium chloride is 63g / L.
[0028] The purification solution is concentrated and crystallized by evaporation, and barium chloride crystals are precipitated, and barium chloride crystals and high-calcium liquid are obtained b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| aspect ratio | aaaaa | aaaaa |
| whiteness | aaaaa | aaaaa |
| aspect ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com