Medicinal application of corylin
A technology of psoralenin and bone fat, applied in the field of natural medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
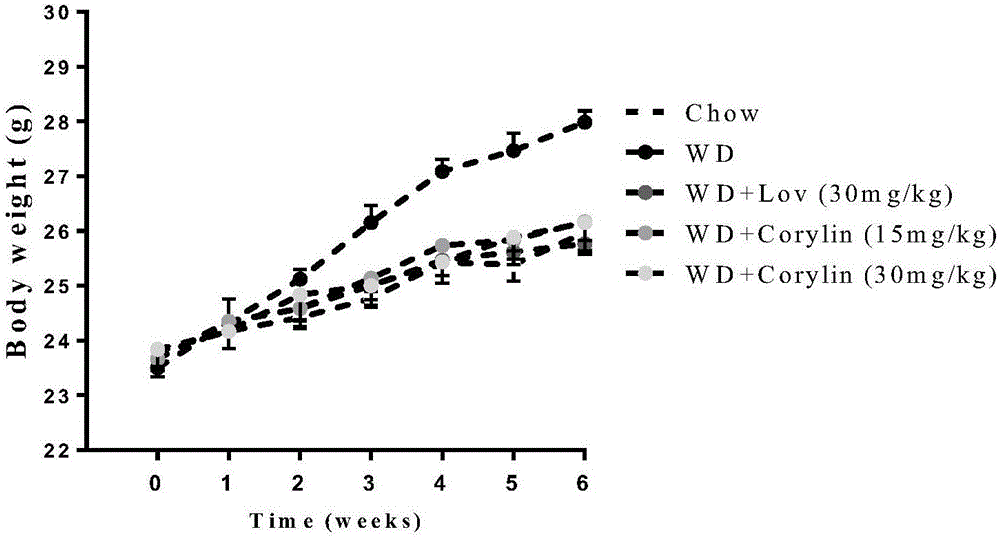
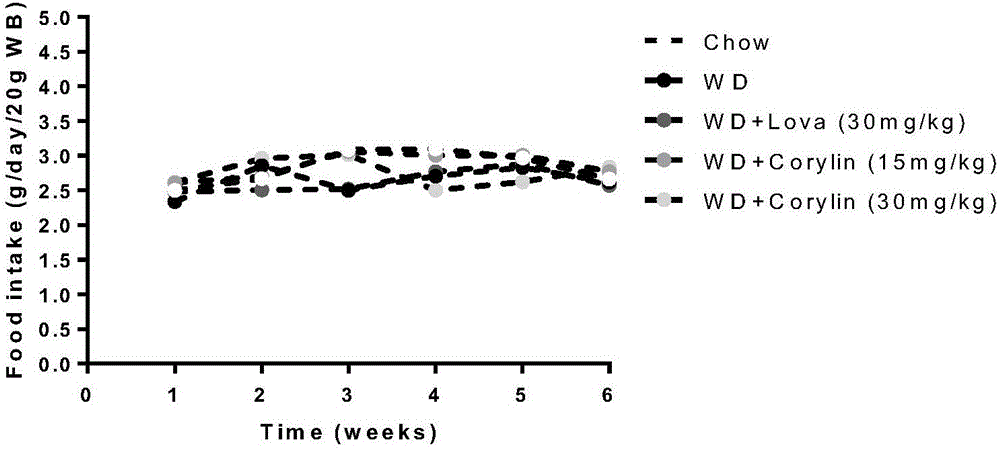
[0046] In order to study the effect of psoralening on the mouse model induced by high fat, use C57BL mice, SPF grade, male, body weight (20 ± 2) g, and randomly divide them into two groups, the first group of 12 is the normal group, Give normal feed, and the remaining mice (40) are divided into 4 groups, give high-fat feed (feed calorie 19.4% protein, 20.6% carbohydrate, 60% fat) free to eat and drink. The matrix, lovastatin group (30mg / kg), psoralenin low concentration group (15mg / kg) and psoralenin high concentration group (30mg / kg) were administered respectively. Continuous feeding for 6 weeks.
[0047] The configuration method of Bakuchining: dissolve with 0.5% CMC-Na solution.
[0048] Observation indicators:
[0049] a general condition of the animal
[0050] The experimental animals were exposed to light for 12 hours and night for 12 hours, free to eat and drink, and the animals were in normal state during the experiment.
[0051] b Observe food intake and body weig...
Embodiment 2
[0056] Effects of Bakuzhining on Animal Models of Hyperlipidemia
[0057] Experimental animals and methods:
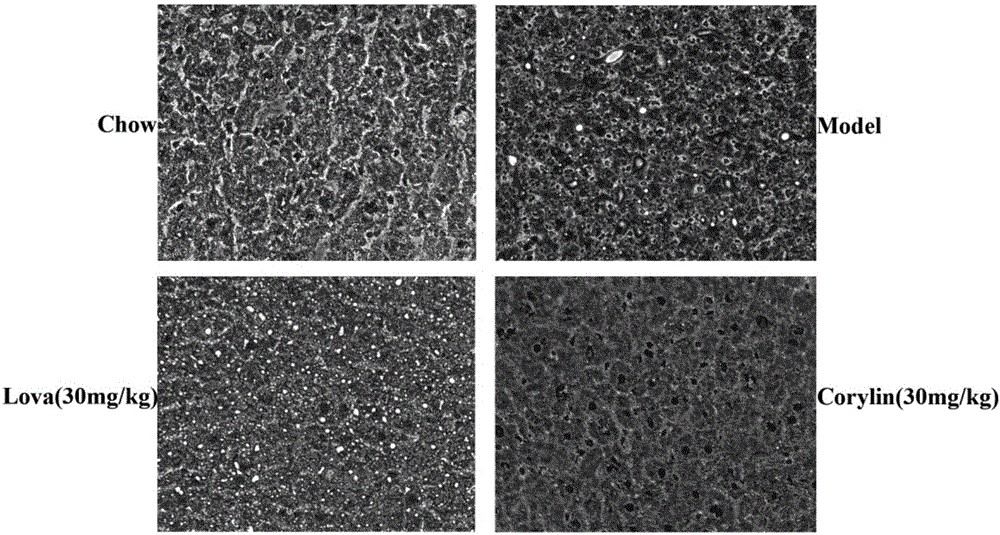
[0058] C57BL mice, SPF grade, male, body weight (20 ± 2) g, were randomly divided into two groups, the first group of 12 was the normal group, given normal feed, and the remaining mice (40) were divided into 4 groups, given With high-fat feed (feed caloric 19.4% protein, 20.6% carbohydrate, 60% fat) ingest and drink freely. The matrix, lovastatin group (30mg / kg), psoralenin low concentration drug (15mg / kg) and psoralenin high concentration group (30mg / kg) were administered respectively. Continuous feeding for 6 weeks. Blood collection, execution, and liver tissue collection.
[0059] Observation indicators:
[0060] a general condition of the animal
[0061] The experimental animals were raised in the SPF animal room, with 12 hours of light and 12 hours of darkness, free to eat and drink, and the animals were in normal state during the experiment.
[0062] b blood ...
Embodiment 3
[0080] Effects of Bakuzhining on Animal Models of Hyperglycemia
[0081] In order to study the effect of psoralening on diabetes, the classic high-fat-induced diabetic mouse model was used in this experiment. C57BL mice, SPF grade, male, body weight (20±2) g, were randomly divided into two groups, the first group 12 Only, be normal group, give normal feed, all the other mice (40) are divided into 4 groups, give high-fat feed (feed caloric 19.4% protein, 20.6% carbohydrate, 60% fat) ad libitum intake, drinking water. The matrix, lovastatin group (30mg / kg), psoralenin low concentration drug (15mg / kg) and psoralenin high concentration group (30mg / kg) were administered respectively. Continuous feeding for 6 weeks. Fasting overnight after the end of administration, fasting blood glucose, glucose tolerance and insulin tolerance were measured. Glucose tolerance test method: mice were fasted overnight, and mice were orally administered with blood glucose 2g / kg at 0, 15, 30, 60, 90, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com