A method for improving drug stability of bivalirudin
A bivalirudin and stability technology, which is applied in the field of preparation of bivalirudin pharmaceutical preparations, can solve the problems of slow product dissolution, inconspicuous improvement, complicated operation, etc., to reduce the risk of medication, improve uniformity, and reduce degradation The effect of chance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
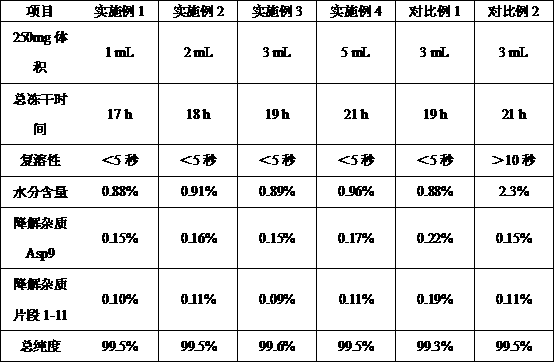
[0017] Embodiment 1, a method for improving the stability of bivalirudin drug: making the bivalirudin bulk drug into a freeze-dried liquid, and then preparing the bivalirudin freeze-dried powder by ultra-low temperature vacuum freeze-drying; before drying Use 1.0 mol / L sodium bicarbonate solution to adjust the pH of the lyophilized liquid to 5.0; during the ultra-low temperature vacuum freeze-drying process: the pre-freezing temperature is -188°C~-55°C, the sublimation drying temperature is -40°C~10°C, and the analysis The drying temperature is 10°C~60°C.
Embodiment 2
[0018] Embodiment 2, a method for improving the stability of bivalirudin drug: making the bivalirudin bulk drug into a freeze-dried liquid, and then preparing the bivalirudin freeze-dried powder by ultra-low temperature vacuum freeze-drying; before drying Use 2.0 mol / L sodium bicarbonate solution to adjust the pH of the lyophilized liquid to 6.0; during the ultra-low temperature vacuum freeze-drying process: the pre-freezing temperature is -188°C~-60°C, the sublimation drying temperature is -30°C~5°C, and the analysis The drying temperature is 20°C~50°C.
Embodiment 3
[0019] Embodiment 3, a method for improving the stability of bivalirudin drug: making the bivalirudin bulk drug into a lyophilized liquid, and then preparing a bivalirudin lyophilized powder injection by an ultra-low temperature vacuum freeze-drying method; before drying Use 1.5 mol / L sodium bicarbonate solution to adjust the pH of the lyophilized liquid to 5.5; during the ultra-low temperature vacuum freeze-drying process: the pre-freezing temperature is -188°C~-70°C, the sublimation drying temperature is -20°C~5°C, analyze The drying temperature is 10°C~50°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com