Difluoro-bithiophene polymer as well as preparation method and application thereof to FET (field effect transistor)
A technology of polymers and dibromo compounds, applied in the fields of electric solid devices, semiconductor devices, semiconductor/solid-state devices, etc., can solve the problems of high LUMO energy level of polymers, unfavorable electron injection into the transport layer, and weak electron withdrawing ability. , to achieve the effect of easy electron injection, high yield and improved planarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1, polymer P1FIID-2FBT (formula I-1)
[0081] The reaction equation is as Figure 6 shown.
[0082] 1) 6-Bromo-1-(4-decyltetradecyl)indole-2,3-dione
[0083] Add 6-bromoisatin (2.00g, 8.85mmol), potassium carbonate (2.93g, 21.24mmol), 1-iodo-4-decyltetradecane (4.93g, 10.62mmol) into a 100mL two-necked flask, 20mL Anhydrous N,N-dimethylformamide, 20mL anhydrous tetrahydrofuran, protected by argon. Reaction at 70°C for 12h. Extract with water and dichloromethane, dry. The solution was spin-dried and passed through a column (eluent: petroleum ether: dichloromethane = 2:1) to obtain 4.09 g of an orange solid. Yield: 82.1%.
[0084] The structural characterization data are as follows:
[0085] Mass Spectrum: HR-MALDI-TOF:[M+Na] + calcd for C32H 52 BrNNaO 2 :584.30794,found:584.30746.
[0086] H NMR and C NMR: 1 H NMR (400MHz, CDCl 3 )δ7.46(d,J=8.0Hz,1H),7.28(dd,J 1 =8.0Hz,J 2 =1.2Hz, 1H), 7.06(d, J=1.2Hz, 1H), 3.67(t, J=7.6Hz, 2H), 1.66(m, 2H), 1.4...
Embodiment 2
[0109] Embodiment 2, polymer P2FIID-2FBT (formula I-2)
[0110] The reaction equation is as Figure 6 shown.
[0111] 1) 6,6'-dibromo-7,7'-difluoro-1,1'-bis(4-decyltetradecyl)isoindigo (Formula 2)
[0112] Add 6-bromo-7-fluoro-1-(4-decyltetradecyl)indole-2,3-dione (0.61g, 1.06mmol) to a 100mL two-necked flask, 6-bromo-7- Fluoro-1-(4-decyltetradecyl)indol-2-one (0.60g, 1.06mmol), p-toluenesulfonic acid (20.2mg, 0.106mmol), 20mL acetic acid, protected by argon. React at 120°C for 12h. Extract with water and dichloromethane, dry. The solution was spin-dried and passed through a column (eluent: petroleum ether: dichloromethane = 6:1) to obtain 0.70 g of a red solid. Yield: 58.7%.
[0113] The structural characterization data are as follows:
[0114] Mass Spectrum: HR-MALDI-TOF:[M+Na] + calcd for C 64 h 102 Br 2 f 2 N 2 NaO 2 :1151.61537,found:1151.61487.
[0115] H NMR and C NMR: 1 H NMR (400MHz, CDCl 3 )δ8.89(d, J=8.4Hz, 2H), 7.19(m, 2H), 3.90(t, J=7.2Hz, 4H), 1....
Embodiment 3
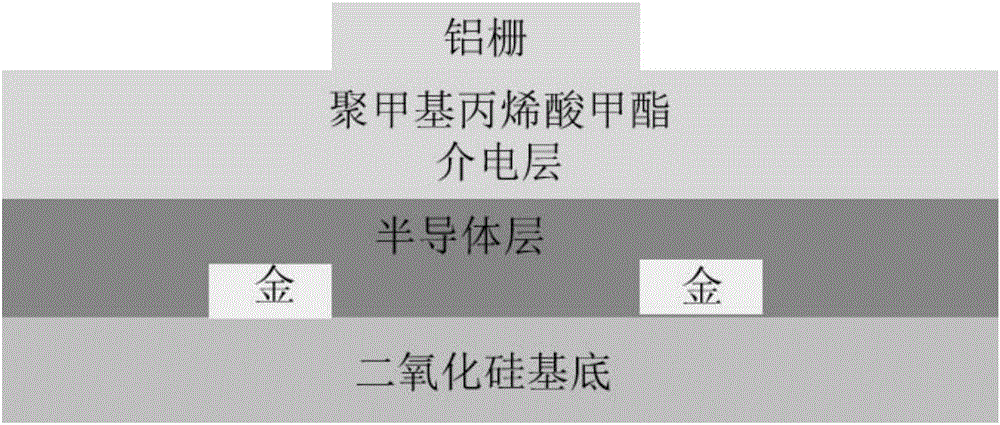
[0123] Embodiment 3, the spectral performance of polymer P1FIID-2FBT and P2FIID-2FBT, electrochemical performance and field effect transistor performance
[0124] 1) Spectral and electrochemical properties of polymers P1FIID-2FBT and P2FIID-2FBT
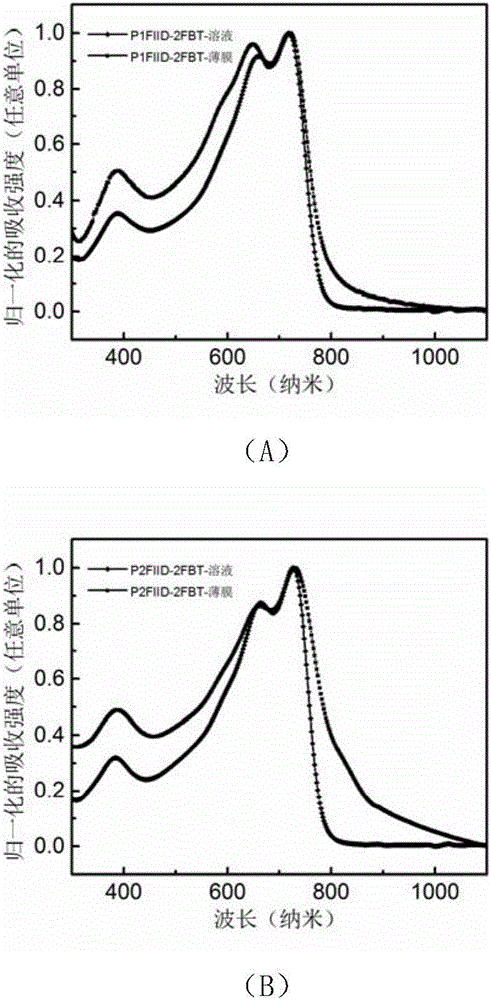
[0125] figure 1 For the polymer P1FIID-2FBT ( figure 1 (A)) and P2FIID-2FBT ( figure 1 (B) UV-Vis absorption spectra in solution and film.
[0126] Depend on figure 1 It can be seen that the optical bandgaps of the polymer films P1FIID-2FBT and P2FIID-2FBT are 1.59eV and 1.40eV respectively (the optical bandgaps are based on the formula E g =1240 / λ calculation, where E g is the optical band gap, and λ is the boundary value of the UV absorption curve). Depend on figure 1 It can be seen that both polymers have relatively strong intramolecular charge transfer peaks, indicating that the intermolecular forces of the polymers are relatively strong.
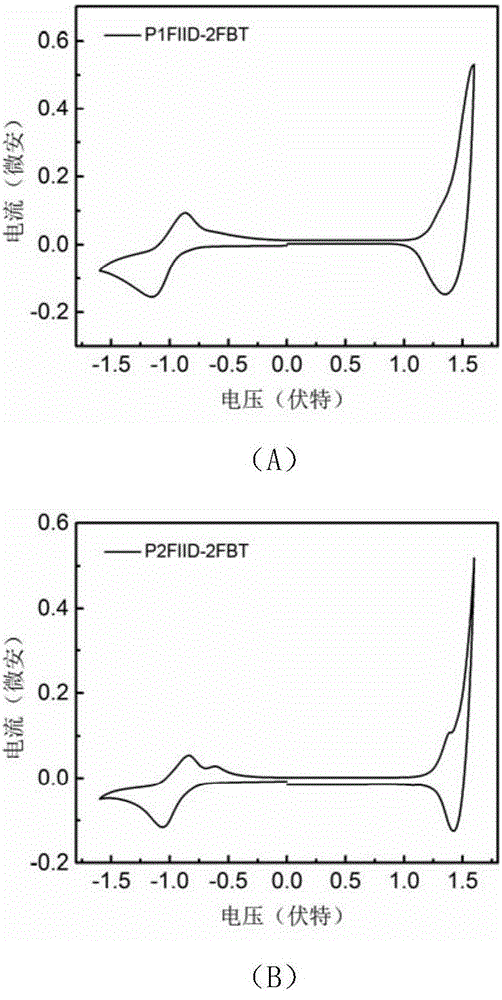
[0127] figure 2 For the polymer P1FIID-2FBT ( figure 2 (A)) and P2FIID-2FBT ( ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical bandgap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com