Metalloporphyrin-quinoxaline organic semiconductor material and preparation method and application thereof
An organic semiconductor, metalloporphyrin technology, applied in semiconductor/solid-state device manufacturing, semiconductor devices, organic chemistry, etc., can solve the problems of limiting the application scope of organic semiconductor materials, no literature and patent reports, etc., to achieve good thermal stability and Environmental stability, improved carrier mobility, and improved utilization effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
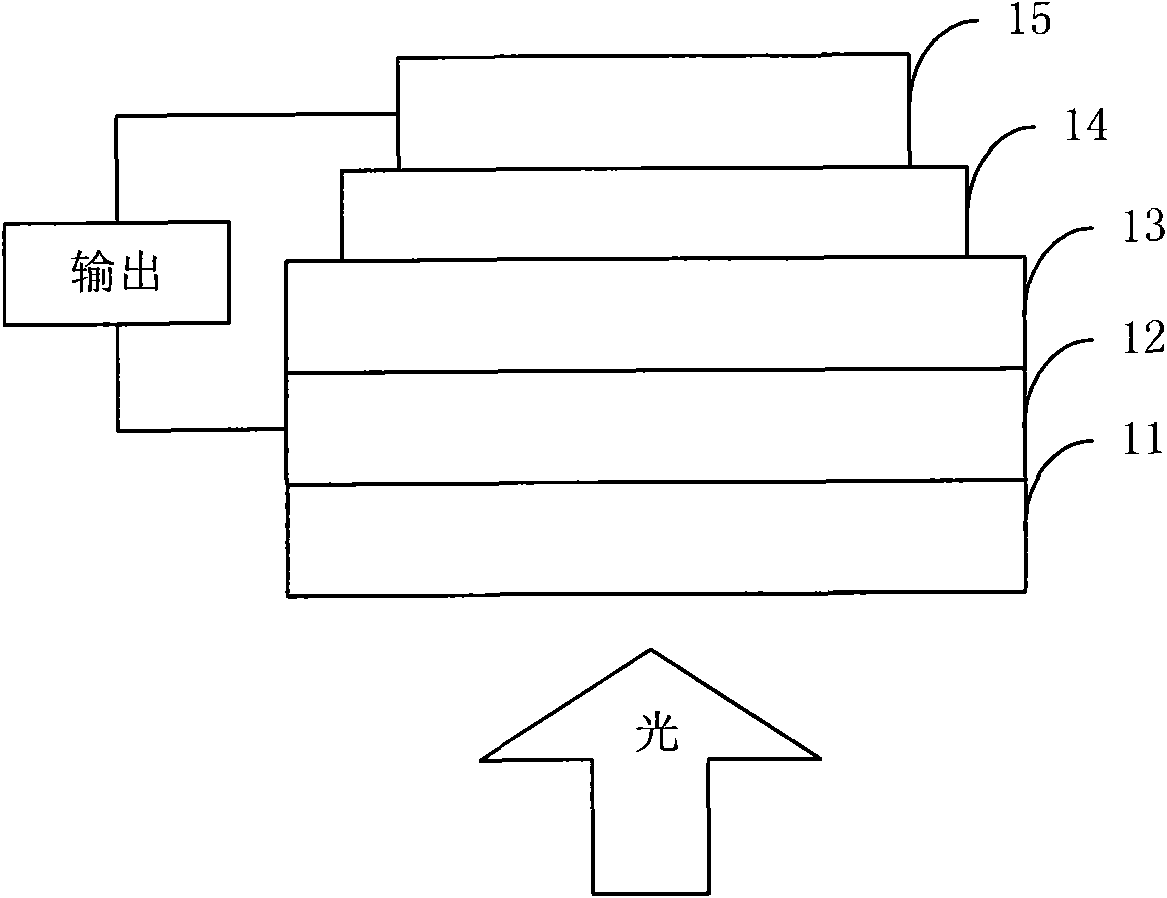
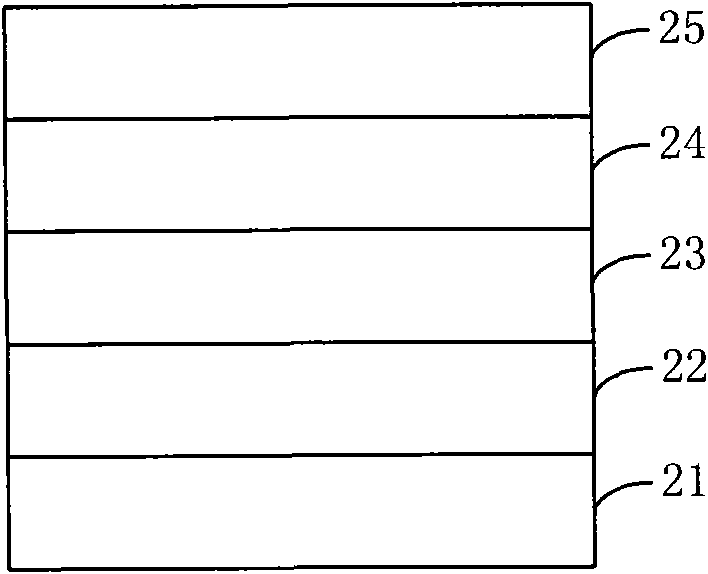
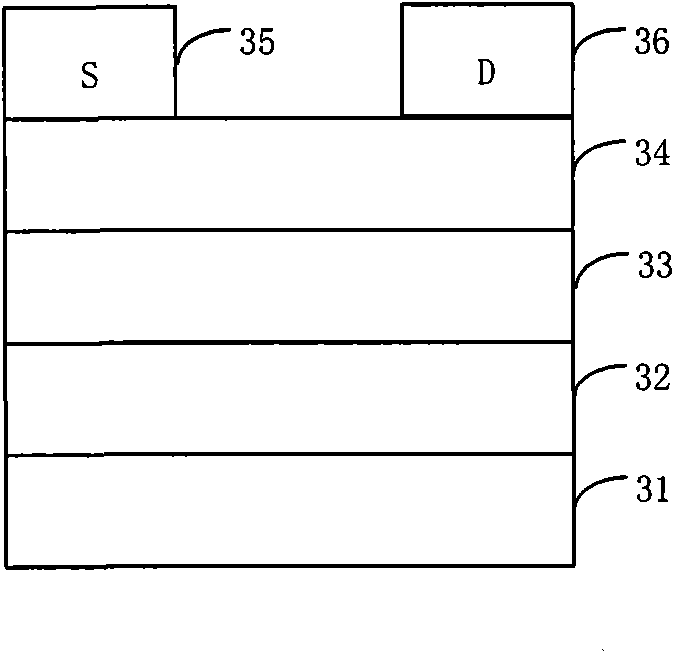
Image
Examples
preparation example Construction
[0045] The preparation method of metalloporphyrin-quinoxaline organic semiconductor material designed by the present invention, the steps are as follows:
[0046] Step S1: Dissolving dipyrromethane (A), the first silafluorene derivative (B) and the second silafluorene derivative (C) in a molar ratio i:j:k in the first organic compound containing the oxidizing agent and the first catalyst In the solvent, add an oxidizing agent and a first catalyst to the first organic solvent, and react at 20-100° C. for 1-24 hours to obtain a silfluorene porphyrin derivative (D), the reaction formula is as follows:
[0047]
[0048] Wherein, i:j:k=1:1~100:1~100, and i=j+k, i≥j>0; the first catalyst adopts organic acid, such as propionic acid, trifluoroacetic acid; the oxidizing agent can adopt two Chlorodicyanobenzoquinone (DDQ), the first organic solvent can be one or both in chloroform or dichloromethane;
[0049] Step S2: Dissolve the silicon fluorene porphyrin derivative (D) obtained i...
Embodiment 1
[0069] This embodiment discloses a silicon fluorene zinc porphyrin-quinoxaline organic semiconductor material with the following structure
[0070]
[0071] In the above formula, n=10;
[0072] The preparation steps of the above-mentioned organic semiconductor material are as follows:
[0073] 1. Synthesis of 5,8-dibromo-2,3-bis(phenyl)quinoxaline
[0074]
[0075]3,6-Dibromo-o-phenylenediamine (1.01 g, 3.7 mmol) was added to a solution of the compound diphenylethanedione (0.39 g, 1.8 mmol) in 20 ml of acetic acid at 120°C. Reflux overnight, pour the reaction solution into water, and neutralize to neutral with sodium bicarbonate. Extracted with chloroform, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was removed by rotary evaporation, and the crude product was obtained by column chromatography as a white solid. Then recrystallized from chloroform / n-hexane to obtain a white solid powder with a yield of 86%.
[0076] GC-MS (EI-m / z)...
Embodiment 2
[0097] This embodiment discloses a silicon fluorene iron porphyrin-quinoxaline organic semiconductor material with the following structure
[0098]
[0099] In the above formula, n=28;
[0100] The preparation steps of the above-mentioned organic semiconductor material are as follows:
[0101] One, the synthesis of 5,8-bis(tributyltin)-2,3-bis(phenyl)quinoxaline
[0102] Its preparation sees embodiment 1 for details.
[0103] 2. Synthesis of 5-(9'-methyl-9'-hexadecyl)silafluorene-15-(9'-docosyl)silafluorene porphyrin
[0104]
[0105] Set up an anhydrous and oxygen-free device, weigh the intermediates 2-aldehyde-9-methyl-9-hexadecylsilafluorene (0.45g, 1mmol), 2-aldehyde-9-docosylsilafluorene ( 0.66g, 1mmol), dipyrromethane (0.30g, 2mmol), dissolved in 250ml of dichloromethane, blown nitrogen for 30min, added 2ml of trifluoroacetic acid into the syringe, stirred at 100°C for 1h, then added dichlorodicyanobenzene Quinone (DDQ) (1.82g, 8mmol), continue stirring at room...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Sheet resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com