Electrical activity recording method for tiny single synaptic neurons
A single-synapse and neuron technology, applied in the scientific field, can solve problems such as the inability to distinguish electrical signals, and achieve the effect of simple and easy-to-use real-time detection methods and techniques
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of brain slice:
[0047] After the surgical instruments are prepared, decapitate the mice of P2-3 (2 days to 3 days after birth), and remove the brainstem with surgical scissors, ophthalmic scissors, scalpel and tweezers, and dip into the Slicing solution from time to time during this process (125 sodium chloride, 25 sodium bicarbonate, 3 inositol, 2 sodium pyruvate, 2.5 potassium chloride, 1.25 sodium dihydrogen phosphate, 0.4 ascorbic acid, 25 glucose, 0.1 calcium chloride, 3 magnesium chloride (mM, sigma), pH 7.4, with 95% oxygen and 5% carbon dioxide). Then the tissue was glued to the slicing chamber, and the brain slices with a thickness of 200 μM were cut out with a microtome. According to the age of the mouse, we can cut 1-3 brain slices containing Medial Nucleus of the Trapezoid Body (MNTB), and the process of cutting the brain slices is also carried out in this slicing solution. Keep on ice. Low Ca levels during brain slice prep...
Embodiment 2
[0048] Example 2: Whole-cell clamping
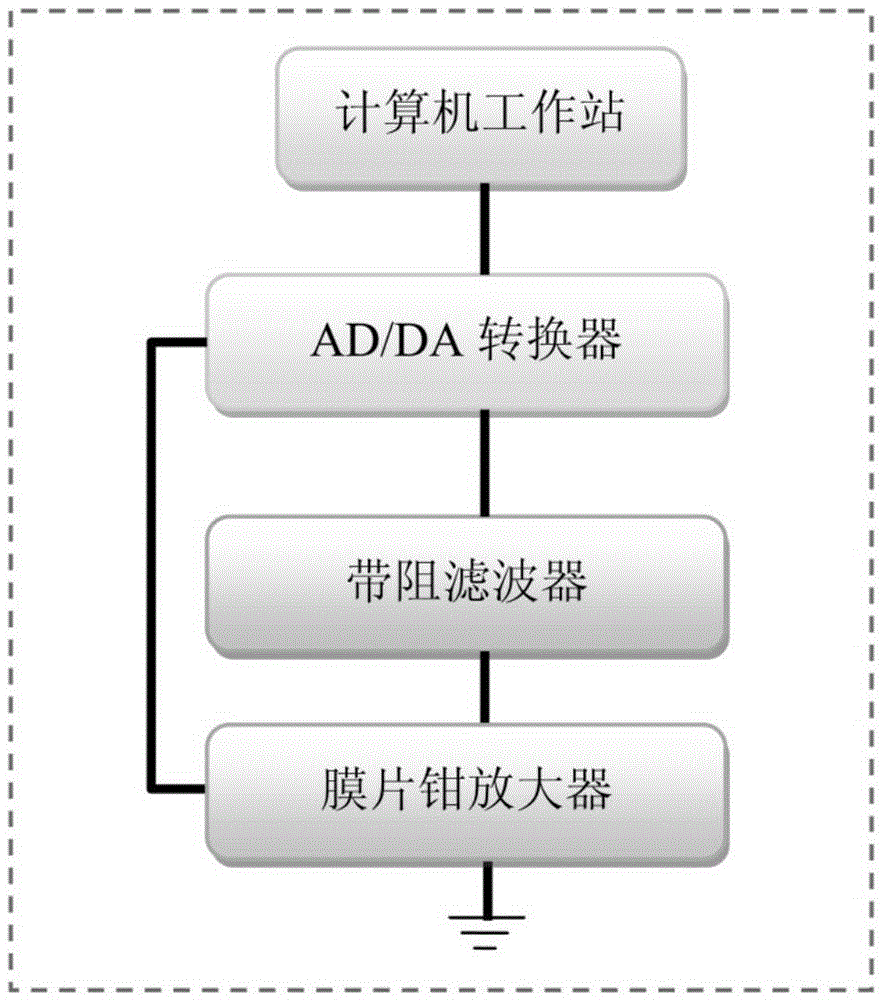
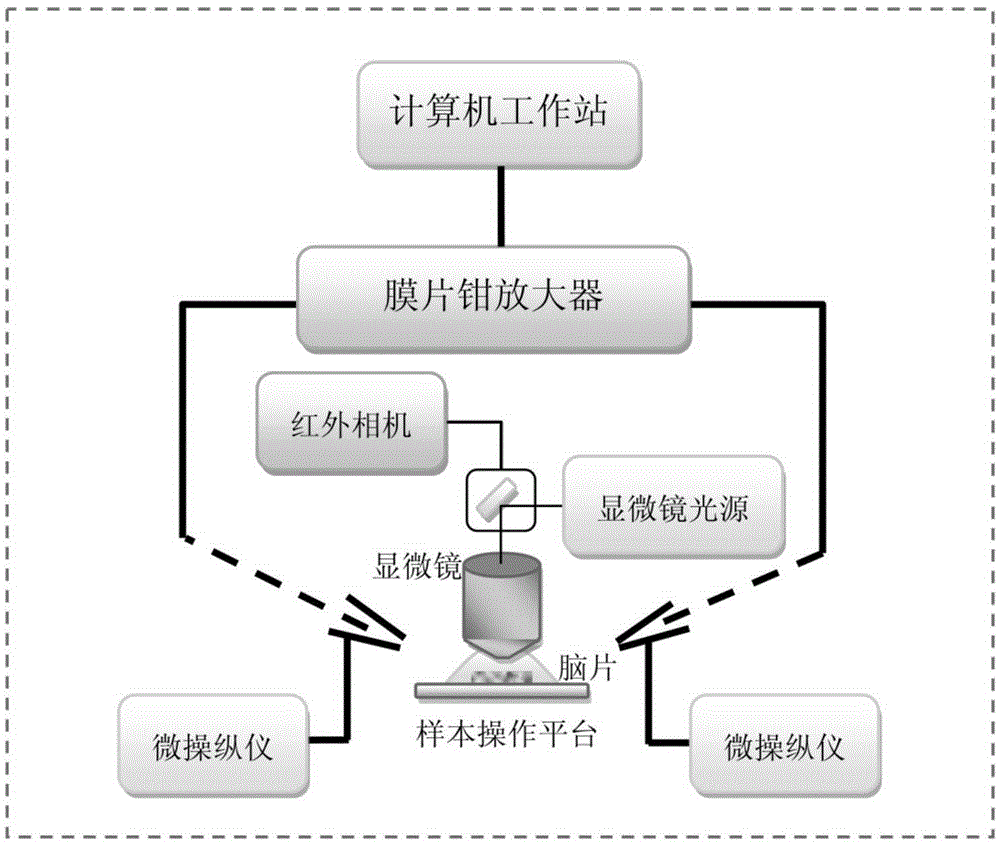
[0049] After the brain slices were incubated, quickly put a piece of brain slice on the glass slide, and cover the brain slice with a platinum U-shaped board wrapped with uniform and equidistant nylon threads (Johnson&Johnson REACH), so that the brain slice was in the There will be no movement on the slide. During the whole experiment, the brain slice was perfused with extracellular fluid (Bath solution) at a rate of 1 ml / min. Also make sure that the space above the slide does not allow extracellular fluid to overflow. Then observe with a microscope, first find the calyx of Held synapse and post-synaptic cell body with a 5× low-power lens, and then observe with a 60× diving lens. After finding the cell body with tiny monosynaptic presynaptic cells, this was clamped whole-cell with a glass microelectrode on one side, and the subsequent recording mode was performed by the MultiCalmp 700B amplifier.
Embodiment 3
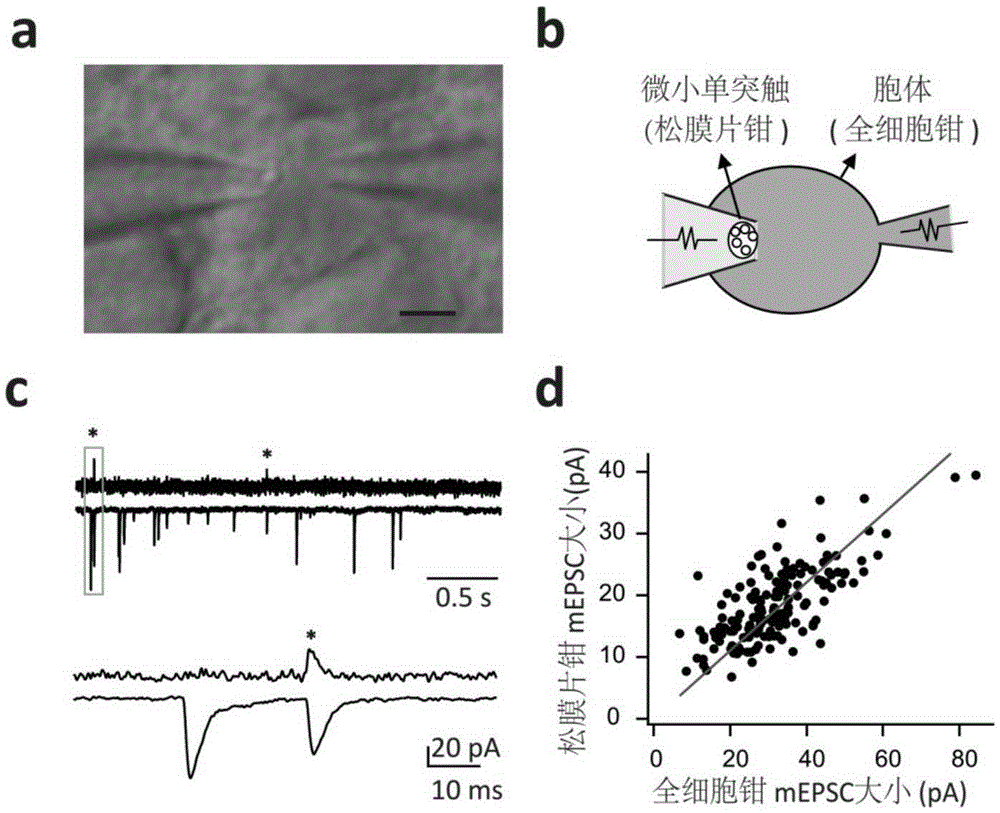
[0050] Example 3: Loose-patch clamping
[0051] After the whole-cell pattern is formed, use the glass microelectrode on the other side to perform loose-patch on the observed tiny monosynaptic presynaptic cells, and the whole process should be very gentle (such as figure 2 a, b shown). Simultaneous recordings of loose patch-clamp channels and whole-cell channels are then possible. Such as figure 2 As shown in c, the signals in the upper figure are from the loose patch-clamp channel and the whole-cell channel respectively, which contain the signal of micro-post-synaptic current (mEPSC); Positive-going electrical signals in patch-clamp recording mode were only highly time- and amplitude-correlated with certain mEPSCs in whole-cell recordings (e.g. figure 2 d), which shows that the recorded mEPSC signal originates from this tiny monosynaptic junction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com