A novel dipropoxyphenyl-methanesulfonamide compound for regulating estrogen-related receptor activity and its medical use
A compound, estrogen technology, used in organic chemistry, amide active ingredients, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
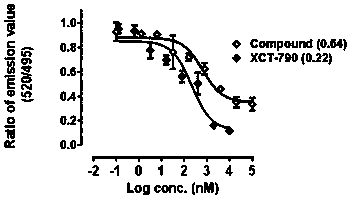
[0025] Example 2 The detection technology of time-resolved fluorescence resonance energy transfer (Time-Resolved Fluoresen-ce Resonance Energy Transfer assay, TR-FRET assay) was used to test the competitive binding activity of the compound of formula I of the present invention to ERRα at the molecular level
[0026]This example illustrates that the compounds involved in the present invention can effectively inhibit the combination of ERRα and PGC1α, indicating that the compounds involved in the present invention competitively bind to ERRα (molecular level). TR-FRET is a technique familiar to those skilled in the art. FRET (Fluorescence Resonance Energy Transfer), that is, fluorescence resonance energy transfer, is based on the energy transfer of two fluorophores (donor and acceptor), when the two fluorophores are close to each other. Interactions between biomacromolecules can be measured with fluorescent labels and energy transfer between the two. When the two groups are clos...
Embodiment 3
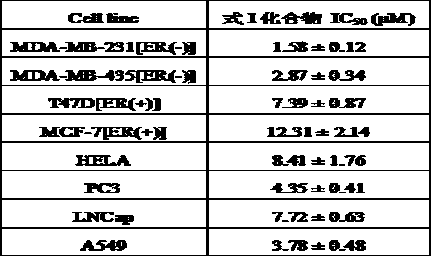
[0030] Example 3 Using CCK-8 Cell Proliferation Assay to Determine the Proliferation Inhibitory Effects of Compounds on Various Tumor Cell Lines
[0031] This example illustrates that the compounds involved in the present invention can effectively inhibit the proliferation of various tumor cell lines, indicating that the compounds involved in the present invention exhibit effective anti-tumor activity in vitro. The CCK-8 cell proliferation assay is a technique familiar to those skilled in the art.
[0032] (1) Tumor cell culture and seeding in 96-well cell culture plate: Take out the tumor cells in the logarithmic growth phase in good condition, observe the cell morphology, if the cells have been 80% confluent, start passage. Wash and discard the old culture medium in the petri dish, wash with sterile 1mL PBS for 3-5 times, add 1mL of warmed trypsin, shake the dish horizontally to make the trypsin evenly distributed and fully digested. Put the cells in the incubator for 1-2 m...
Embodiment 4
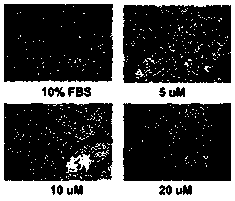
[0037] Example 4 Using Transwell tumor cell migration assay to determine the effect of compounds on the migration function of breast cancer cell MDA-MB-231
[0038] This example illustrates that the compounds involved in the present invention can effectively inhibit the migration of breast cancer cells MDA-MB-231, indicating that the compounds involved in the present invention exhibit effective anti-migration activity of breast cancer cells in vitro. Transwell tumor cell migration assay is a technique familiar to those skilled in the art.
[0039] (1) Preparation of Transwell chamber: Matrigel 1:8 (50mg / L) dilution was used to coat the upper chamber surface of the bottom membrane of the Transwell chamber to coat the basement membrane, and air-dried at 4°C. After air-drying, hydrate the basement membrane, suck out the residual liquid in the culture plate, add 50uL serum-free DMEM culture solution containing 10g / L BSA to each well, and incubate in a 37°C incubator for 30min.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com