Micro-nano material, product of covalent modification of hydrophilic substances for surface of micro-nano material, and preparation method
A covalent modification, micro-nano technology, applied in the preparation of organic compounds, carbamic acid derivatives, sulfonic acid, etc., can solve the problems of reducing reactivity and modification degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
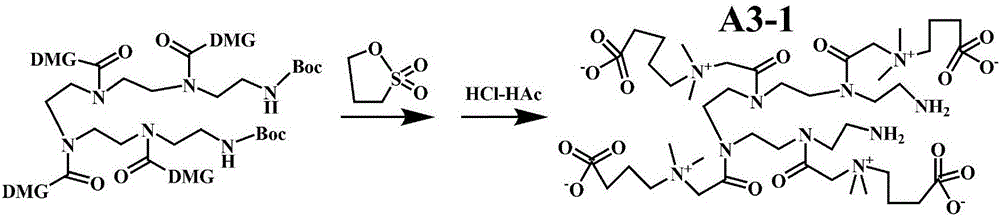
[0093] The invention also discloses a method for preparing the product of covalently modifying the surface of the micro-nano material with a hydrophilic substance, comprising the following steps:
[0094] a. Generate carboxyl active ester on the surface of the micro-nano material, that is, use the micro-nano material with carboxyl or / and potential carboxyl on the surface as the micro-nano material to be modified, and convert the carboxyl or / and potential carboxyl on its surface into an active ester; The micro-nano material refers to an organic polymer with a size of at least one dimension not exceeding 100 μm, a composite of an organic polymer and an organic or inorganic micro-nano material, and has two shapes; one is a particle and the particle size is less than 100 μm , which can be spherical and approximately spherical; the other is a thin film with a thickness of less than 100 μm but does not limit the area size of this thin film, including free thin films or thin films att...
Embodiment 1
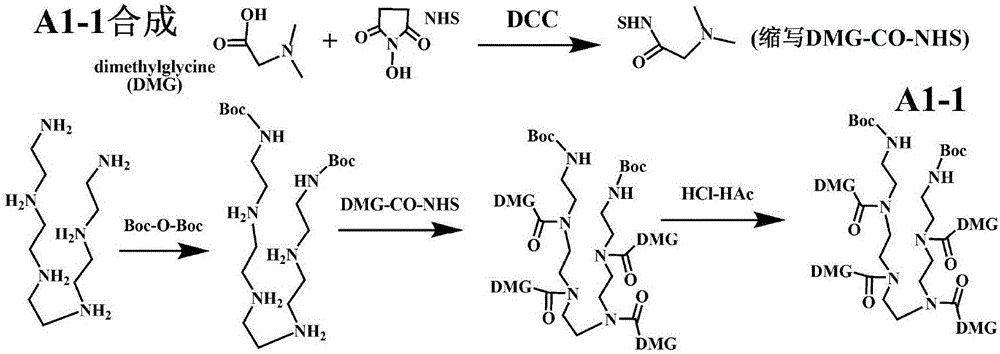
[0202] Example 1. Preparation of Representative Type A1-1 Modifiers
[0203] 1-1. Synthesis of N,N-dimethylglycine active ester: in tetrahydrofuran (THF), saturated dimethylglycine (DMG) plus NHS and DCC (the molar ratio of the three is 1:1:1), and the reaction was stirred overnight at room temperature; The precipitate was filtered off, the supernatant was concentrated 20 times under reduced pressure, and ether was added to precipitate the precipitate; then dissolved in the minimum amount of hot THF, and ether was added to precipitate the precipitate. In N, N-dimethylglycine active ester (DMG-CO-NHS).
[0204] 1-2. Pentaethylene hexamine bis-Boc protected primary amine synthesis: pentaethylene hexamine is saturated dissolved in dimethylformamide (DMF), and double the molar amount of bis-tert-butyl pyrocarbonate (Boc-O-Boc ), stirred and reacted at room temperature for 60 minutes; added diethyl ether to precipitate a precipitate; redissolved in the minimum amount of DMF, added...
Embodiment 2
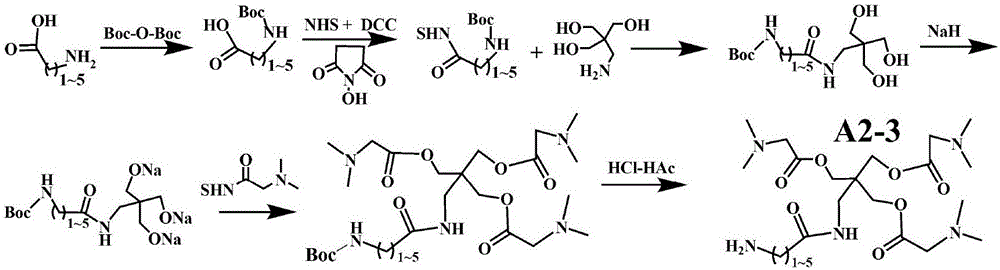
[0207] Example 2. Preparation of Representative Type A2-3 Modifiers
[0208] 2-1. Synthesis of active esters of N-Boc protected amino acids: straight-chain amino acids with amino groups within 6 carbons at the end (glycine, 3-aminopropionic acid, 4-aminobutyric acid, 5-aminopentanoic acid, 6-aminocaproic acid) 1) Saturated and dissolved in water at room temperature, adding twice the molar amount of bis-tert-butanol pyrocarbonate (Boc-O-Boc) saturated and dissolved in THF, stirring at room temperature for 2.0 hours; adding isopropanol until a large amount of precipitates precipitated; Re-dissolve to the minimum amount of DMF, add ether to precipitate the precipitate; repeat twice to obtain N-Boc protected amino acid. Finally, saturate the N-Boc protected amino acid dissolved in DMF, add DCC and NHS (molar ratio 1:1:1); stir at room temperature overnight. Filter off the precipitate, add ether to the supernatant to precipitate the precipitate; redissolve in the least amount of h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com