4-Aminoquinoline compound and preparation method thereof
A technology for aminoquinoline and compounds, applied in the field of 4-aminoquinoline compounds and their preparation, can solve the problems of low yield, limited sources of raw materials, complicated reaction process, etc., and achieve cheap raw materials, easy access to raw materials, and simple methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention also provides a kind of preparation method of 4-aminoquinoline compound, the method comprises:
[0042] Step 1: Under acidic conditions, react dithioketenes with arylamines, or react enaminones, aryl isothiocyanates and halogenated hydrocarbons under alkaline conditions to obtain imino-N , S-ketene compound;
[0043] Step 2: The imino-N,S-ketal compound obtained in Step 1 is subjected to a self-cyclization reaction under the catalysis of a copper salt to obtain a 4-aminoquinoline compound.
[0044]Specifically, to prepare 4-aminoquinoline compounds with the structure of formula I, the preparation method preferably includes:
[0045] Under acidic conditions, dithioketal and arylamine react to generate imino-N, S-ketal compound; the molar ratio of said dithioketal to arylamine is preferably 1:(2- 3). The acidic conditions used preferably include Lewis acid, Bronsted acid, superacid, or acid modified by immobilization. More preferably boron triflu...
Embodiment 1
[0054] 1) Synthesis of imino-N,S-ketal compound 3
[0055]
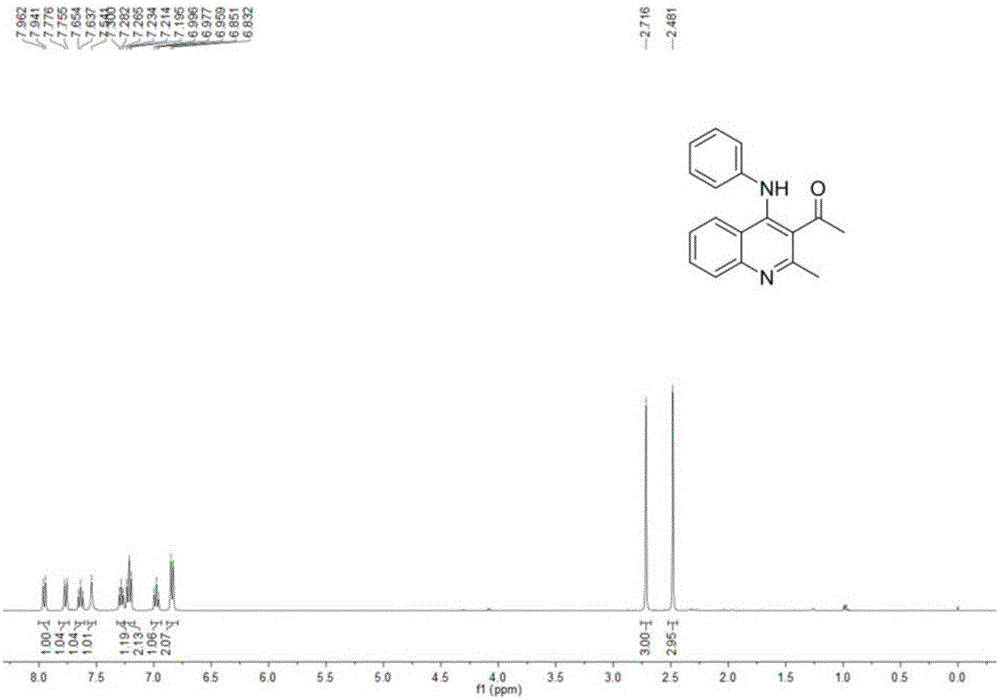
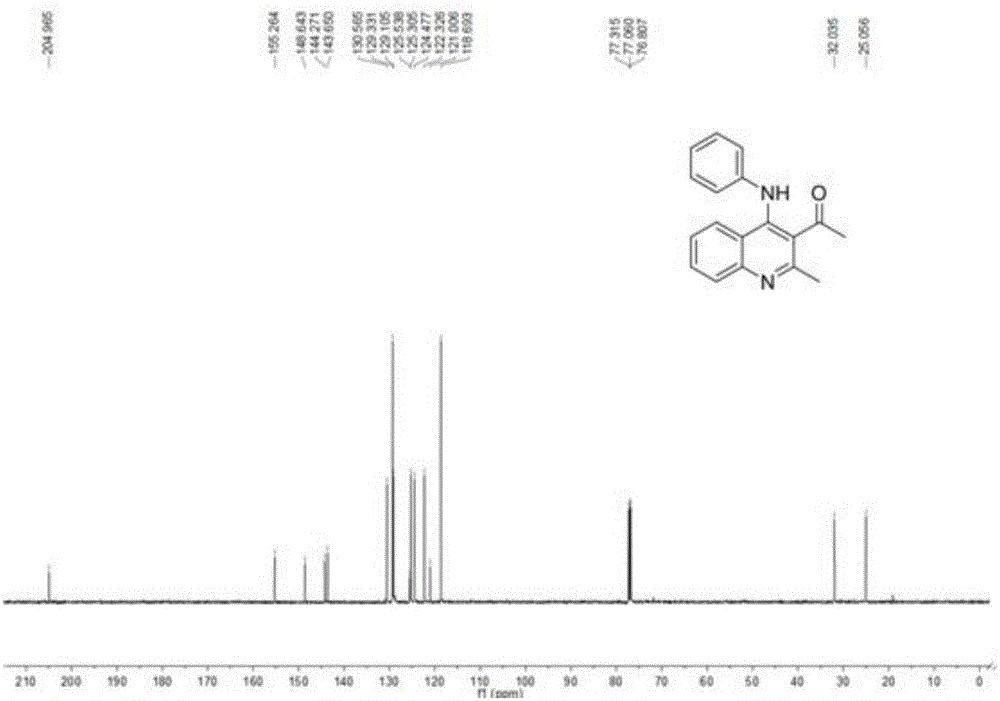
[0056] Into a 50 mL round bottom flask equipped with a magnetic stirring device was added α-acetyl-α-carboethoxydithioketene 1a (2.62 g, 10 mmol), aniline 2a (2.79 g, 30 mmol), toluene 20 mL and trifluoro Boronium diethyl ether (0.37mL, 3mmol), stirred at 80 ° C, thin layer chromatography (TLC) to monitor the end of the reaction (5h), the reaction solution was carefully poured into saturated sodium chloride solution (20mL), dichloromethane (3 × 20mL) extraction, merging organic phases, drying over anhydrous sodium sulfate, suction filtration, vacuum distillation and other steps to obtain viscous solids, through silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =60:1) 2.39 g of a viscous yellow solid was obtained. The structure of the product was confirmed by NMR and mass spectrometry to be imino-N,S-ketal compound 3a, and the yield was 65%.
[0057] 2) Synthesis of 4-aminoquinoline compound 4a
[0058] ...
Embodiment 2
[0063] Replace the aniline in "Example 1" with 4-chloroaniline, replace 1a with benzylthio substituted dithioketene ketal, and other conditions are the same as "Example 1". The experimental results are shown in Table 1.
[0064]
[0065] Spectrum analysis 4b:
[0066] 1 H NMR (500MHz, CDCl 3 )δ8.47(s,1H),7.83(d,J=9.0Hz,1H),7.63(s,1H),7.55(d,J=9.0Hz,1H),7.20(d,J=8.5Hz, 2H), 6.81(d, J=8.5Hz, 2H), 4.32(q, J=7.0Hz, 2H), 2.77(s, 3H), 1.37(t, J=7.0Hz, 3H). 13 C NMR (125MHz, CDCl 3 )δ168.4, 157.6, 147.0, 147.0, 141.7, 131.6, 130.7, 130.7, 129.2, 127.9, 123.8, 120.7, 114.8, 61.9, 26.1, 14.0. HRMS (ESI-TOF) Calcd for C 19 h 17 Cl 2 N 2 o 2 (M+H) + 375.0662. Found 375.0655.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com