Methods for preparing halogen ethanol and ethylene oxide
A technology of ethylene oxide and haloethanol, which is applied in the preparation of haloethanol, the preparation of ethylene oxide by a new halohydrin method, and the field of preparation of ethylene oxide, can solve the problem of generating dichloroethane, serious corrosion, waste of raw materials, etc. problems, to achieve the effect of less steam consumption, lower biological oxygen content, and load lightening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] 1) Haloalcoholization: Add 70wt% concentration of hydrogen peroxide H 2 o 2 , HCl solution (hydrochloric acid) and ethene of 35wt% concentration, carry out chloroalcoholization reaction at the temperature of 45 ℃, wherein the hydrogen peroxide H of 70wt% concentration 2 o 2 , 35wt% concentration of HCl solution and ethylene flow should make H 2 o 2 The molar ratio of HCl to ethylene is about 1.2:1.2:1. This gives the halohydrin, a mixture of 2-chloroethan-1-ol and 1-chloroethan-2-ol.
Embodiment 2
[0089] 1) Haloalcoholization: add HTS-1 molecular sieve, 35wt% concentration of hydrogen peroxide H in the tower reactor 2 o 2 , HCl solution (hydrochloric acid) and ethylene of 20wt% concentration, carry out chlorohydrinization reaction at the temperature of 35 ℃, wherein the mass ratio of HTS-1 molecular sieve and ethylene is 0.05:1, the hydrogen peroxide H of 35wt% concentration 2 o 2 , 20wt% concentration of HCl solution and ethylene should be added in such a way that H 2 o 2 The molar ratio of HCl to ethylene is about 1.5:1.1:1. This gives the halohydrin, a mixture of 2-chloroethan-1-ol and 1-chloroethan-2-ol.
Embodiment 3
[0097] 1) Haloalcoholization: Add 70wt% concentration of hydrogen peroxide H 2 o 2 , HCl solution (hydrochloric acid) and ethene of 35wt% concentration, carry out chloroalcoholization reaction at the temperature of 45 ℃, wherein the hydrogen peroxide H of 70wt% concentration 2 o 2 , 35wt% concentration of HCl solution and ethylene flow should make H 2 o 2 The molar ratio of HCl to ethylene is about 1.2:1.2:1. This gives the halohydrin, a mixture of 2-chloroethan-1-ol and 1-chloroethan-2-ol.
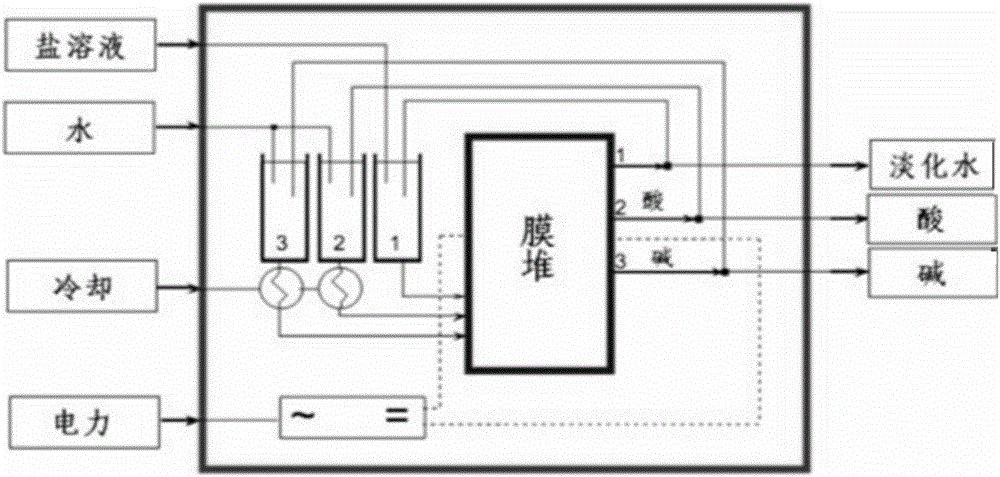
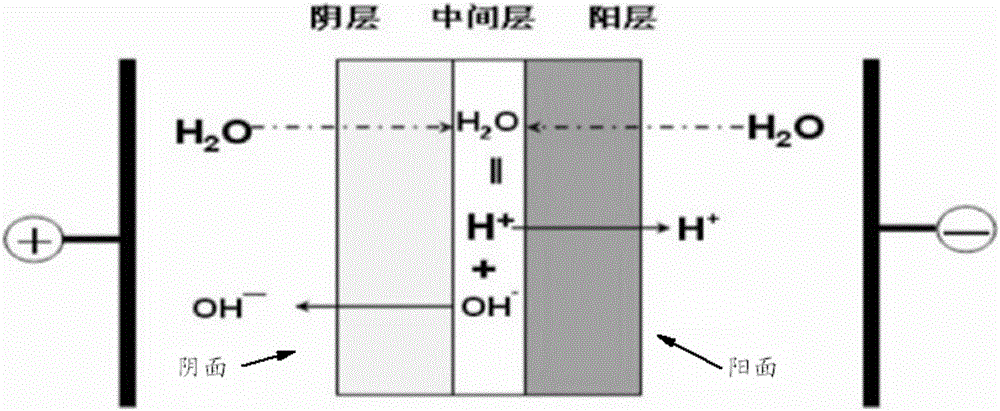
[0098] 2) Saponification: performing saponification reaction with the halohydrin obtained in step 1) and sodium hydroxide, and separating to obtain an ethylene oxide organic phase and a sodium chloride solution. The saponification reaction is carried out in a steel tower reactor, and the upper part is designed as a sieve tray tower. Steam enters from the bottom of the tower to blow out the crude ethylene oxide generated from the top of the tower. The saponification temperature is c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com