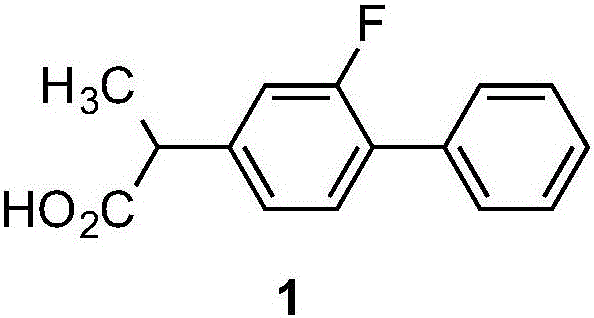
A preparing method of flurbiprofen
A technology of flurbiprofen and molar ratio, which is applied in the field of preparation of flurbiprofen, can solve the problems of great harm to the environment and operators, high cost, low yield, etc., and achieve high atom utilization rate, less three wastes, and low yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
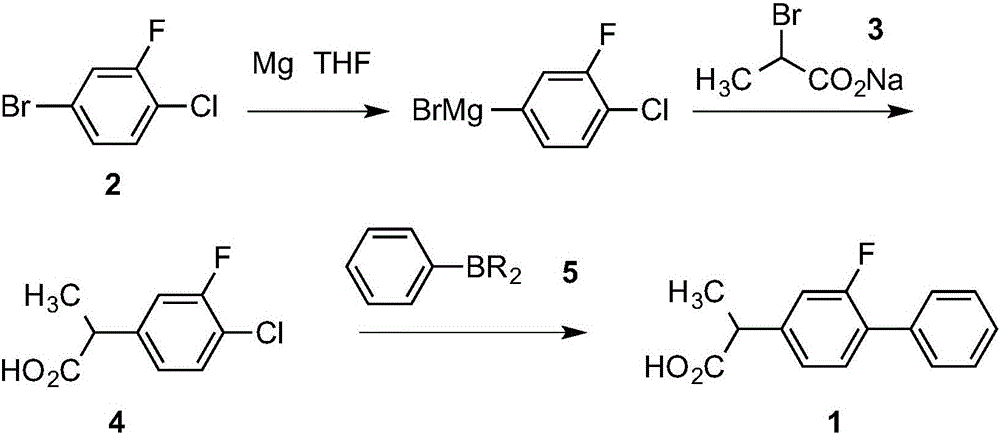
[0033] A, preparation of 2-(3-fluoro-4-chlorophenyl) propionic acid (4)
[0034] In a 1000ml dry four-neck flask, add 14.4g (0.6mol) of magnesium chips, 50ml of THF, and two iodine pellets, stir and heat to 50°C in a nitrogen atmosphere. Dissolve 105g (0.5mol) of 3-fluoro-4-chlorobromobenzene (2) in 370ml of THF and drop it into 20ml. After confirming the trigger and the reaction is stable, add the remaining solution dropwise at 50-60°C, and keep it warm after dropping Stir for 1h.
[0035] Cool the reaction liquid to 0°C, add 96.3g (0.55mol) sodium 2-bromopropionate (3) at a temperature not exceeding 10°C, and gradually heat to reflux for 1 hour after the addition is complete, and the reaction ends.
[0036] Cool the reaction solution to 0°C, slowly add 400ml of 5mol / L hydrochloric acid, the temperature does not exceed 20°C, stir naturally for 30min after the addition, then heat to 50°C and stir for 1h.
[0037] Separate the liquid, keep the organic phase, and extract the a...
Embodiment 2
[0043] Preparation of 2-(2-fluoro-biphenyl-4-yl)propionic acid——flurbiprofen (1)
[0044] In a 1000ml three-necked flask, add 85g (0.42mol) of the product 2-(3-fluoro-4-chlorophenyl)propionic acid (4) and 89.7g of phenylboronic acid pinacol ester (5) in step A of Example 1 (0.44mol), ethanol 200ml, toluene 300ml, potassium carbonate 121g (0.88mol) dissolved in 200ml of water solution. After stirring evenly, add PdCl under the protection of nitrogen 2 (Amphos) 2 155mg (0.05mol%). Heating to reflux for 4h, TLC showed no raw material (4), and the reaction was over.
[0045]The solvent was distilled off, cooled to 0°C, and filtered with suction. The resulting solid was washed with 200 ml of cold water. Then the solid was dissolved in hot water, and hydrochloric acid was added dropwise until the pH value was less than 3, and a large amount of solid was precipitated, which was dried by suction filtration at room temperature to obtain 95.5 g of white flurbiprofen (1), with a yie...
Embodiment 3
[0048] A, preparation of 2-(3-fluoro-4-chlorophenyl) propionic acid (4)
[0049] In a 1000ml dry four-neck flask, add 14.4g (0.6mol) of magnesium chips, 50ml of THF, and two iodine pellets, stir and heat to 50°C in a nitrogen atmosphere. Dissolve 105g (0.5mol) of 3-fluoro-4-chlorobromobenzene (2) in 370ml of THF and drop it into 20ml. After confirming the trigger and the reaction is stable, add the remaining solution dropwise at 50-60°C, and keep it warm after dropping Stir for 1h.
[0050] Cool the reaction solution to 0°C, add 105g (0.6mol) sodium 2-bromopropionate (3) at a temperature not exceeding 10°C, and gradually heat to maintain 50-60°C for 2 hours after the addition, and the reaction is completed.
[0051] Cool the reaction solution to 0°C, slowly add 400ml of 5mol / L hydrochloric acid, the temperature does not exceed 20°C, stir naturally for 30min after the addition, then heat to 50°C and stir for 1h.
[0052] Separate the liquid, keep the organic phase, and extrac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com