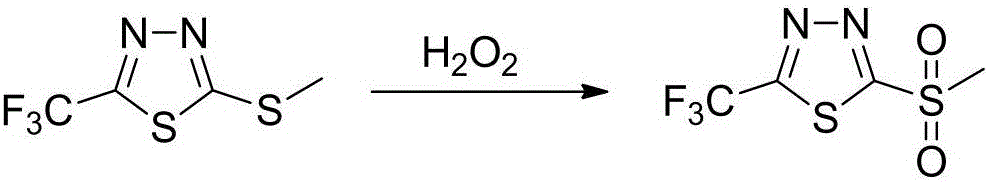Synthetic method of 2-methylsulfuryl-5-trifluoromethyl-1,3,4-thiadiazole
The technology of a trifluoromethyl group and a synthesis method, which is applied in the field of organic synthesis reaction, can solve the problems of unfavorable large-scale industrial production of flufenoxamine, high process risk, potential safety hazard, etc., and achieves low cost, improved reaction efficiency, and pollution. less sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
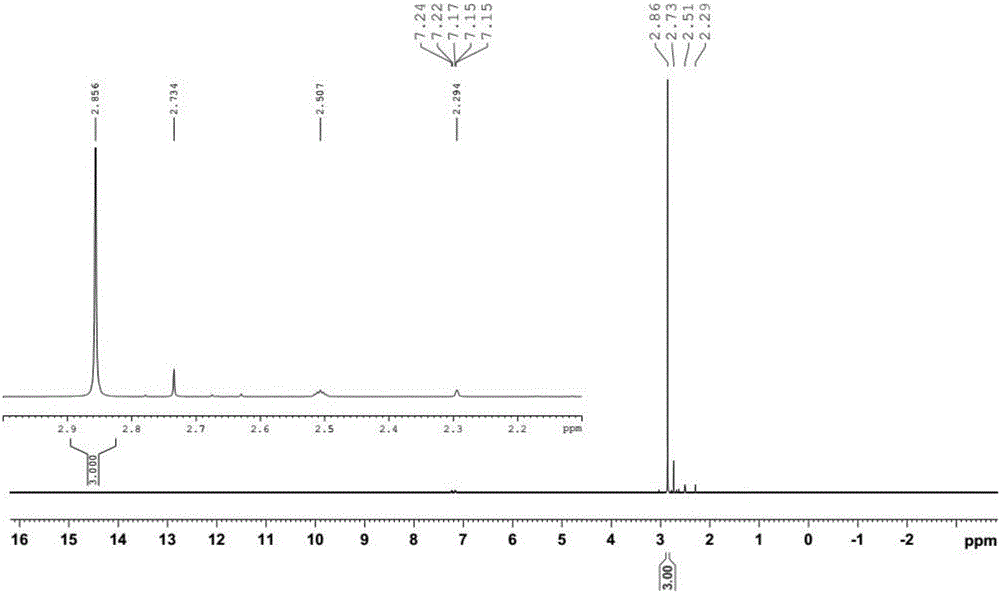
[0029] Add 50.1g (0.25mol, content 99%) of 2-methylthio-5-trifluoromethyl-1,3,4- Thiadiazole, 3.1g acetic acid, 0.02g sodium tungstate, 0.15g tetrabutylammonium chloride, stir and heat up to 53°C, start to add 90.7g (0.8mol, content 30%) hydrogen peroxide dropwise, after the dropwise addition, 54°C Insulate for 5 hours, cool down to 0°C, filter with suction, and dry to obtain snow-white scaly crystals: 2-(methylsulfonyl)-5-(trifluoromethyl)-1,3,4-thiadiazole, purity 98.9 %, yield 96.3%.
Embodiment 2
[0031] Add 100.2g (0.5mol, content 99%) of 2-methylthio-5-trifluoromethyl-1,3,4- Thiadiazole, 10g acetic acid, 0.05g sodium tungstate, 0.11g tetrabutylammonium chloride, stir and heat up to 52°C, start to add 168g (1.5mol, content 30%) hydrogen peroxide dropwise, after the dropwise addition, keep at 55°C for 5h , cooled to -2°C, suction filtered, and dried to obtain snow-white scaly crystals: 2-(methylsulfonyl)-5-(trifluoromethyl)-1,3,4-thiadiazole, purity 99.1% , yield 96.3%.
Embodiment 3
[0033] Add 200.4g (1mol, content 99%) of 2-methylthio-5-trifluoromethyl-1,3,4-thiadiol to a 1000ml four-necked bottle equipped with mechanical stirring, a constant pressure dropping funnel, and a thermometer Oxadiazole, 15g acetic acid, 0.1g sodium tungstate, 0.4g benzyltriethylammonium bromide, stir and heat up to 55°C, start to add 336g (3mol, content 30%) hydrogen peroxide dropwise, after the dropwise addition, keep at 55°C for 6h , cooled to 1°C, suction filtered, and dried to obtain snow-white scaly crystals: 2-(methylsulfonyl)-5-(trifluoromethyl)-1,3,4-thiadiazole with a purity of 99.0%. Yield 96.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com