Combination therapy for treating cancer with a recombinant poxvirus expressing a tumor antigen and an immune checkpoint molecule antagonist or agonist
A technology of immune checkpoints and tumor-associated antigens, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-animal/human immunoglobulin, cancer antigen components, etc., can solve the problem of cancer treatment Meeting medical needs and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0312] Construction of MVA-BN-mHER2
[0313] Simultaneous infection and transfection of cultures allows homologous recombination between the viral genome and the recombinant plasmid. Insert-carrying viruses are isolated, characterized, and virus stocks are prepared.
[0314] Plasmid pBN146 contains sequences that are also present in MVA-BN (14L and 15L open reading frames). The mHER2 sequence was inserted between the MVA-BN sequences to allow recombination into the MVA-BN viral genome. Therefore, a plasmid containing the mHER2 sequence downstream of a poxvirus promoter, in particular the vaccinia type A inclusion body gene promoter, was constructed. This plasmid also contains a selection cassette containing a synthetic vaccinia virus promoter (Ps), a drug resistance gene (guanine-xanthine phosphoribosyltransferase; Ecogpt), an internal ribosome entry site (IRES), and enhanced green fluorescence protein. Two selection genes (gpt and EGFP) are encoded by a single bicistronic...
Embodiment 2
[0331] Increased IFNγ due to treatment with MVA-BN-mHER2 and anti-CTLA4
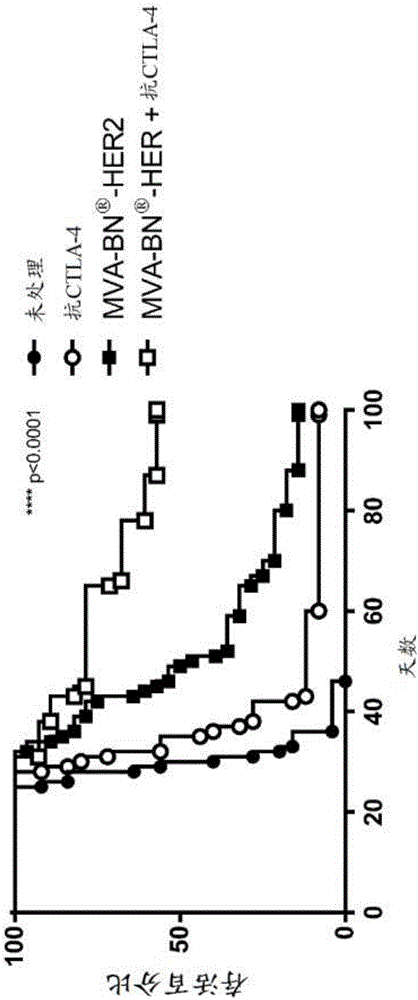
[0332] Female BALB / c mice (6-8 weeks old, approximately 20 g) were purchased from Simonsen Laboratories, Gilroy, CA. For the experimental lung metastasis model, mice were vein grafted on day 1 with 5.0 × 10 4 CT26-HER-2 cells forming tumors in the lungs in 300 μL DPBS.
[0333] The following antibodies were purchased from Bio X Cell (West, Lebanon, NH): anti-ICOS agonistic antibody (clone 17G9), anti-CTLA-4 (9D9), anti-PD-1 (RMP1-14) and anti-LAG-3 ( C9B7W). All antibodies were injected intraperitoneally at 200 μg per mouse in 100 μL PBS on days 3 and 17 unless otherwise indicated. For virus treatment, unless otherwise indicated, 7.1 μL of 1.0 × 10 7 Infection units of MVA-BN-HER2-treated mice.
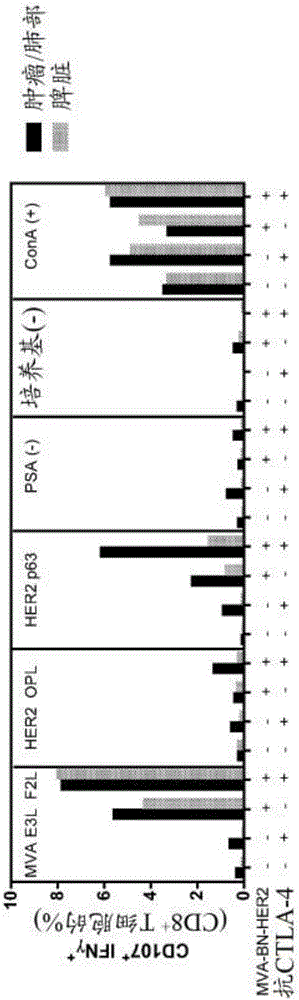
[0334] On day 25, whole blood, tumor / lung or spleen (4 mice / group) were pooled for flow cytometry analysis. Splenocytes were prepared by pressing the spleen between two frosted glass slides and lysing red ...
Embodiment 3
[0342] Increased IFNγ and cytokine production due to treatment with MVA-BN-mHER2 and anti-CTLA4
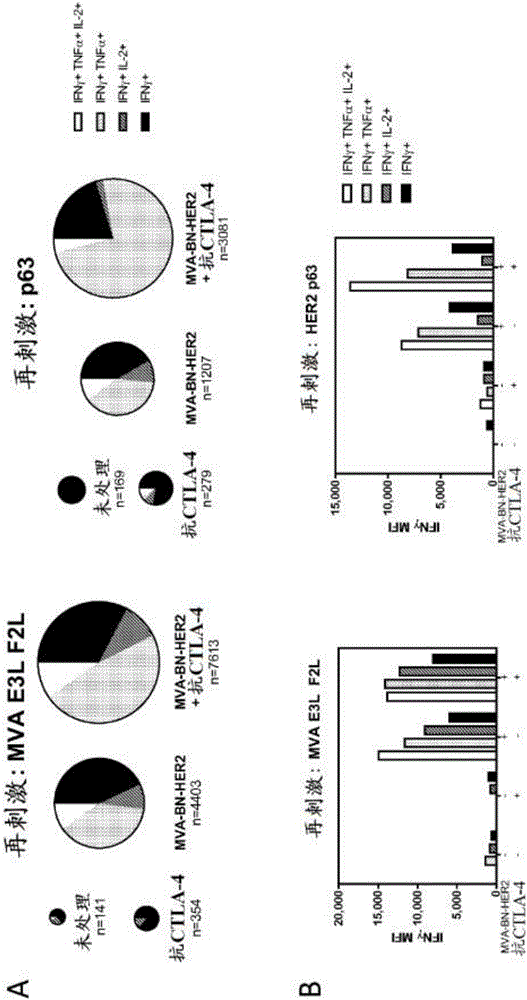
[0343] Treatment with MVA-BN-HER2 increased the quantity and quality of tumor antigen- and virus-specific T cells in the spleen. As described in Example 2, mice were implanted with 5 × 10 4 CT26-HER-2 cells were treated with MVA-BN-HER2 and anti-CTLA4. On day 25, tumor / lung or spleen (4 mice / group) were pooled and restimulated overnight as described in Example 2 to measure virus and tumor antigen specific responses.
[0344] result in figure 2 Shown in , A) Pie charts are area weighted to reflect the number of IFNγ+ cells per million CD8+ T-cells. B) IFNγMFI increases with tumor antigen-specific (HER2p63) multifunctional T cells with combination therapy.
[0345] In both Examples 2 and 3, the number and quality of antigen- and virus-specific T cells increased as a result of combined treatment with MVA-BN-HER2 and anti-CTLA-4. Furthermore, combination treatment increased the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com