AZD 9291 pharmaceutical salt and crystal form and preparation method thereof
A technology of AZD9291, 1.AZD9291, applied in organic chemical methods, drug combinations, anti-tumor drugs, etc., can solve the problems of inconvenient preparation processing operations, high hygroscopicity of mesylate, and poor solvent solubility, and achieves overcoming The effect of high humidity and easy deliquescence, meeting the requirements of bioavailability and efficacy, and facilitating processing operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: AZD9291 Sulfate
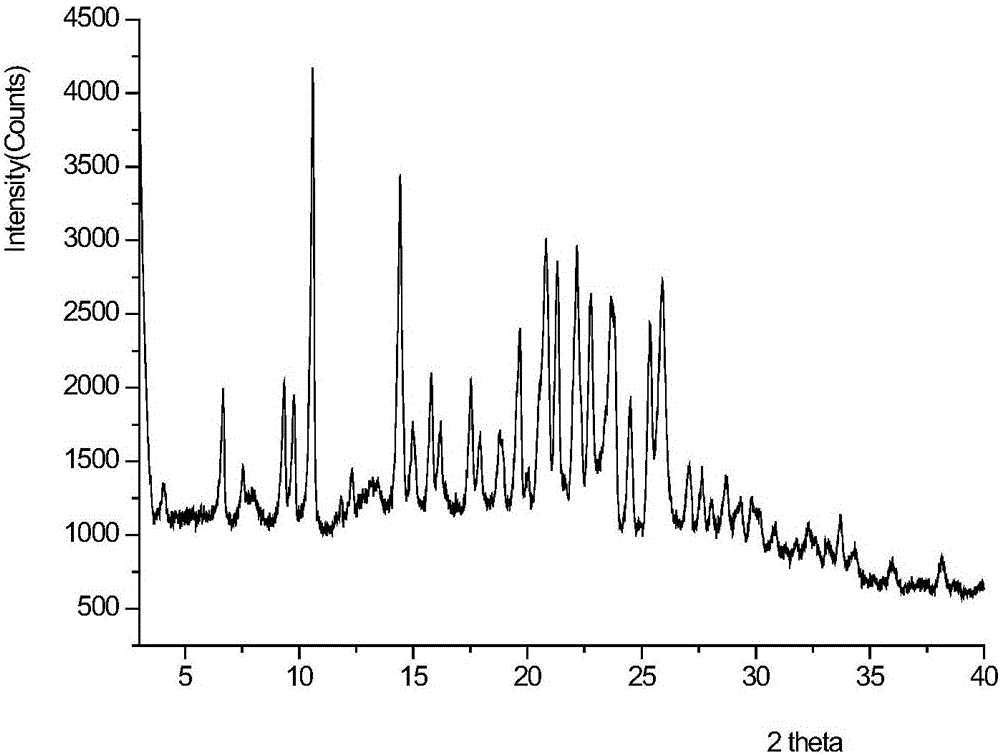
[0061] At room temperature, add AZD9291 (5g, 10mmol, 1.0eq) to the reaction flask, add 50mL of acetone and stir to dissolve, add dropwise an aqueous solution (20mL) of sulfuric acid (1.18g, 12mmol, 1.2eq) under stirring, a yellow solid is precipitated, stir about After half an hour, filter with suction, rinse the filter cake with a small amount of acetone, and dry to obtain 5 g (8.4 mmol) of a yellow granular solid, with a molar yield of 84%. After testing, the purity: 99.4%. Melting point: 255.2°C-257.3°C.
[0062] The AZD9291 sulfate product prepared by the above method, its 1 HNMR such as Figure 5 As shown, the identification data are as follows: 1 HNMR (400MH Z ,DMSO)δ:9.54(1H,s),9.25(1H,s),8.66(1H,s),8.62(1H,s),8.32~8.30(2H,d,J=5.6Hz),7.57~7.55 (1H, d, J=8Hz), 7.31~7.26(2H,m), 7.20~7.16(1H,t,J=7.4Hz), 7.05(1H,s), 6.75~6.68(1H,m), 6.38 ~6.31(1H,m),5.83~5.81(1H,d,J=10.4Hz),3.92~3.91(6H,d,J=5.2Hz),3.33~3.31(4H,m),2.84(6H,s ),2.66(...
Embodiment 2
[0067] Embodiment 2: AZD9291 p-toluenesulfonate
[0068] At room temperature, add AZD9291 (5g, 10mmol, 1.0eq) to the reaction flask, add 50mL of acetone and add TsOH·H 2 O (2.4g, 12mmol, 1.2eq) in aqueous solution (5ml), precipitated a yellow solid, stirred for half an hour, filtered with suction, rinsed the filter cake with a small amount of acetone, and dried to obtain 4.03g (6.0mmol) yellow granular solid, Molar yield 60%. After testing, the purity is 99.7%, and the melting point is 214.0℃~215.6℃.
[0069] The AZD9291 p-toluenesulfonate product prepared by the above method, its 1 HNMR such as Figure 9 As shown, the identification data are as follows: 1 HNMR (400MH Z,DMSO)δ:9.57(1H,s),9.19(1H,s),8.76(1H,s),8.55(1H,s),8.34~8.32(2H,m),8.00(1H,s),7.56 ~7.54(1H,d),7.50~7.48(2H,d),7.28~7.24(2H,m),7.19~7.11(3H,m),7.03(1H,s),6.71~6.64(1H,m) ,6.38~6.33(1H,dd),5.84~5.81(1H,m),3.92~3.91(6H,d),3.32~3.27(4H,m),2.84~2.83(6H,d),2.64(3H, s),2.30(3H,s);ESI-MSm / z:500.3[M+H] + ,250....
Embodiment 3
[0073] Embodiment 3: AZD9291 tartrate
[0074] At room temperature, add AZD9291 (5g, 10mmol, 1.0eq) to the reaction flask, add 50mL of acetone to dissolve, add tartaric acid (1.8g, 12mmol, 1.2eq) aqueous solution (5mL) under stirring, precipitate a light yellow solid, stir for half an hour , suction filtration, the filter cake was rinsed with a small amount of acetone, and dried to obtain 5 g (7.7 mmol) of light yellow granular solid with a molar yield of 77%. After testing: purity 99.9%; melting point: 169.6°C ~ 172.0°C.
[0075] The AZD9291 tartrate product prepared by the above method, its 1 HNMR such as Figure 13 As shown, the identification data are as follows: 1 HNMR (400MH Z ,MeOD)δ:8.75(1H,s),8.36~8.34(1H,d,J=8Hz),8.30~8.29(1H,d,J=5.28Hz),8.23(1H,s),7.49~7.47( 1H,d,J=8Hz),7.28~7.18(3H,m),7.00(1H,s),6.62~6.48(2H,m),5.90~5.87(1H,dd,J=9.6Hz,2Hz), 4.44(2H,s),4.03(3H,s),3.92(3H,s),3.50~3.47(2H,t,J=5.4Hz),3.29~3.26(2H,t,J=5.6Hz),2.88 (6H,s),2.75(3H,s);ESI-MS m / z:500....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com