Black frame toad antioxidant peptide and its gene and its application in pharmaceuticals
A toad and black frame technology, applied in the field of biomedicine, can solve the problems of insufficient research on skin active peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1, black box toad antioxidant peptide gene cloning:
[0032] 1. Extraction of total RNA from black-framed toad skin: the living black-framed toad was cleaned with water, put into liquid nitrogen for quick freezing for 4h, got skin tissue, weighed, got 300mg skin tissue, added 10m total RNA extraction buffer (Trizol solution, the U.S. GIBCOBRL company product), homogenized in 20m1 glass homogenizer for 30min. Add an equal volume of phenol / chloroform solution, mix vigorously, place at room temperature for 10 minutes, centrifuge at 12,000 rpm for 10 minutes at 4°C, and discard the precipitate. Add an equal volume of isopropanol to the supernatant, place at room temperature for 10 minutes, centrifuge at 12,000 rpm for 10 minutes at 4°C, wash the precipitate once with 75% ethanol, and dry it. The precipitate at the bottom of the tube is the total RNA of black-framed toad skin.
[0033] II. Purification of mRNA from black-framed toad skin: the isolation and purificat...
Embodiment 2
[0045] Example 2, the preparation of black frame toad antioxidant peptide:
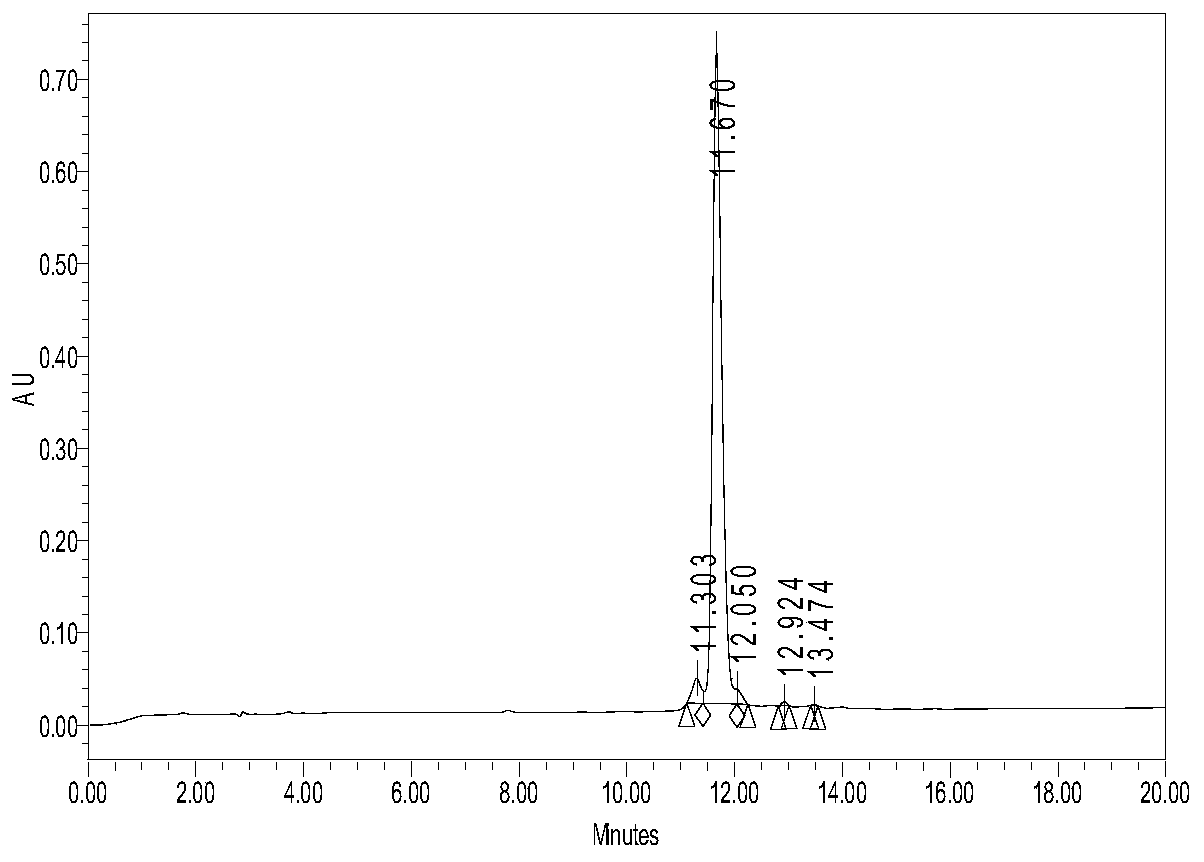
[0046] Ⅰ. The preparation method of black-frame toad antioxidant peptide: According to the gene of black-frame toad antioxidant peptide, the amino acid sequence of the mature active secreted peptide encoding the function is deduced, and then the peptide is synthesized by an automatic polypeptide synthesizer. The formation of the disulfide bond adopts the air oxidation method, specifically dissolving the polypeptide in a flask at 0.1 mg / ml in 0.1% acetic acid solution, titrating with ammonium hydroxide to pH 7.8, and then stirring overnight at room temperature. Desalted and purified by HPLC reverse phase C18 column chromatography. Liquid A is 0.05% TFA+2% CH during purification 3 CN, liquid B is 0.05% TFA+90% CH 3 CN, the concentration gradient of solution B is 20-40% in 15 minutes, the detection wavelength is 220nm, and the polypeptide appears at 11.670 minutes.
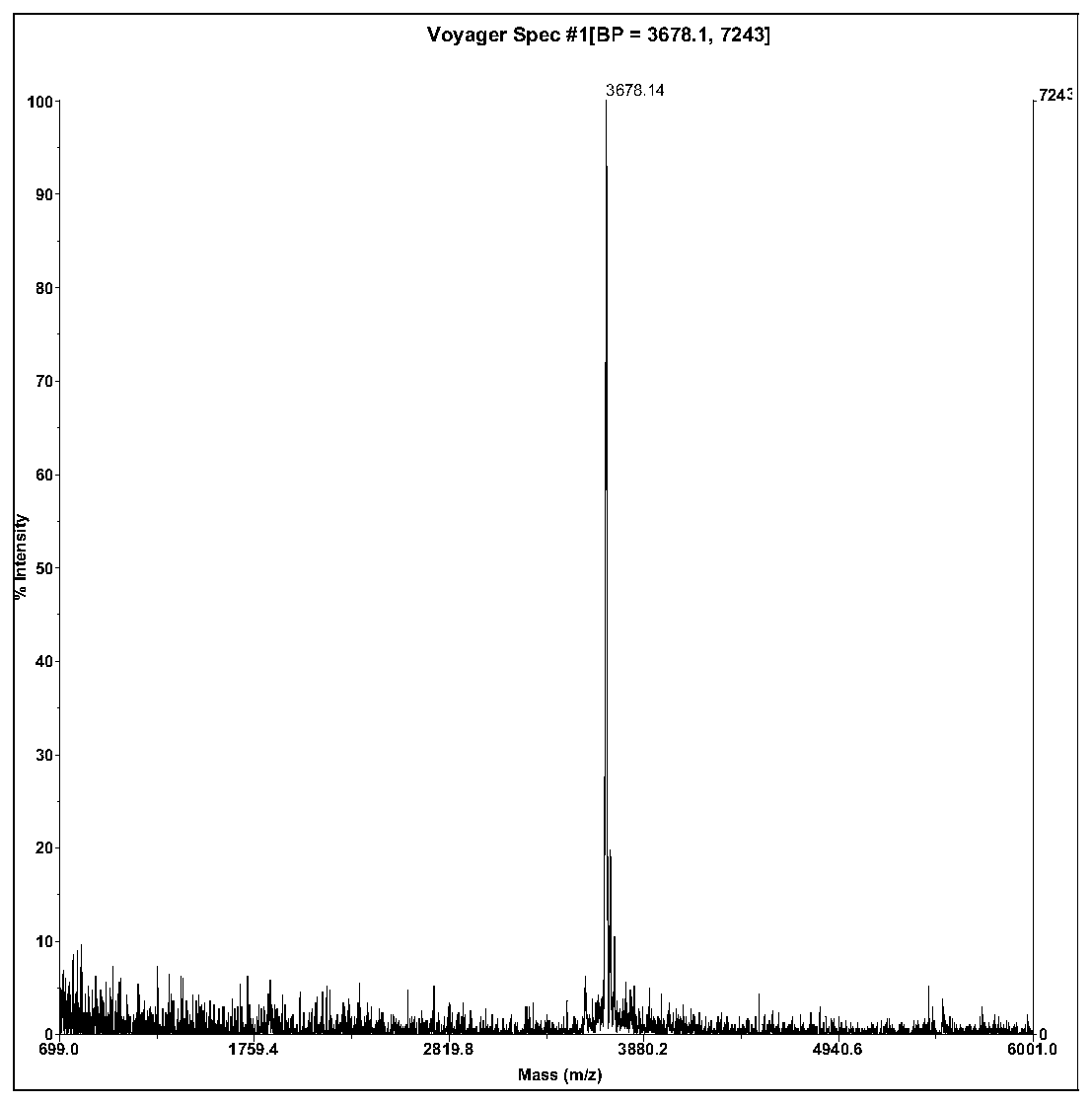
[0047] Ⅱ. The molecular weight is ...
Embodiment 3
[0050] Example 3, the activity experiment of black box toad antioxidant peptide
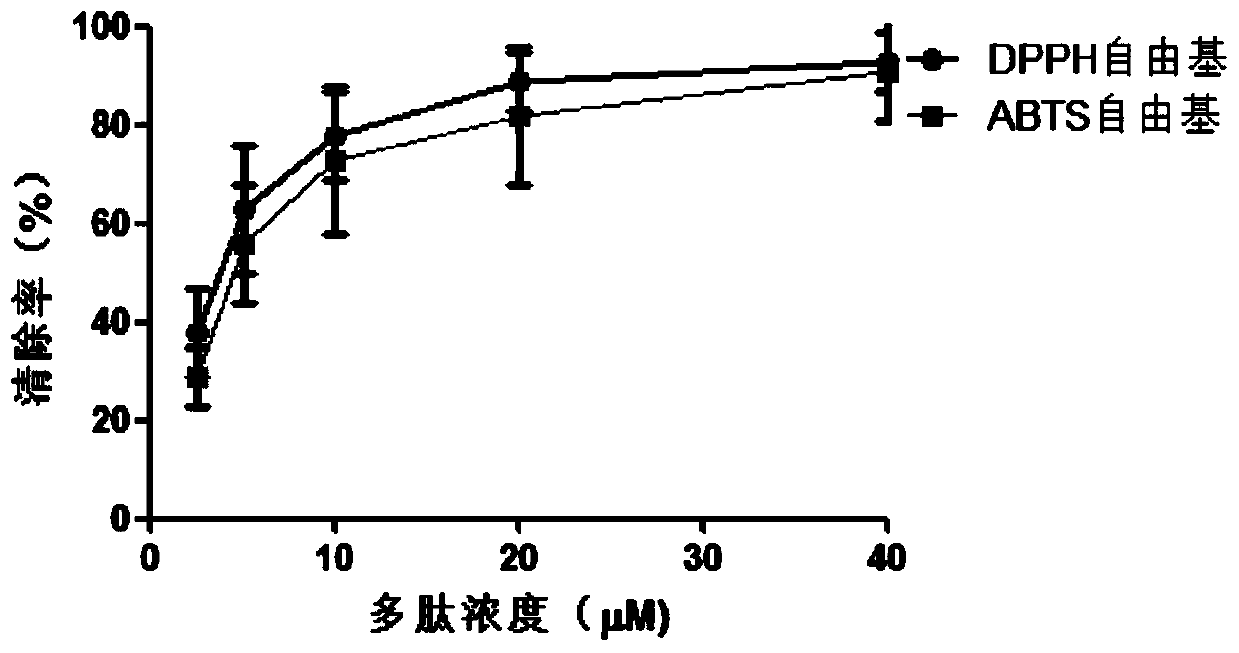
[0051] Ⅰ. Determination of antioxidant capacity
[0052] 1) Determination of DPPH free radical scavenging ability
[0053] DPPH (1,1-diphenyl-2-picryl-hydrazyl) free radical scavenging rate assay was used to study antioxidant peptides. Prepare a DPPH ethanol solution with a concentration of 1×10-5 mol / L, and store it in the dark. Add 2ml, 0.1mM DPPH absolute ethanol solution into a clean test tube containing 2ml of different enzymatic hydrolysis samples, and mix well. After standing at room temperature for 30 minutes, measure the absorbance at 517nm, the smaller the absorbance value, the stronger the ability to scavenge free radicals.
[0054] Clearance (%)={1-(A i -A j ) / A 0}*100%
[0055] In the formula, A 0 2ml, 0.1mM DPPH absolute ethanol solution + 2ml sample reagent, blank control, A i 2ml, 0.1mM DPPH absolute ethanol solution + 2ml sample, A j 2ml of absolute ethanol + 2ml of sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com