Detection kit for CAG trinucleotide duplication mutation of SCA (Spinocerebellar Ataxia) pathogenic genes
A trinucleotide, pathogenic gene technology, applied in the field of molecular biology, can solve the problems of low diagnostic specificity and sensitivity, low specificity, and inability to determine the internal structure of mutant genes well, and achieves mature and reliable technical methods. , The operation steps are simple and the effect of improving the accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
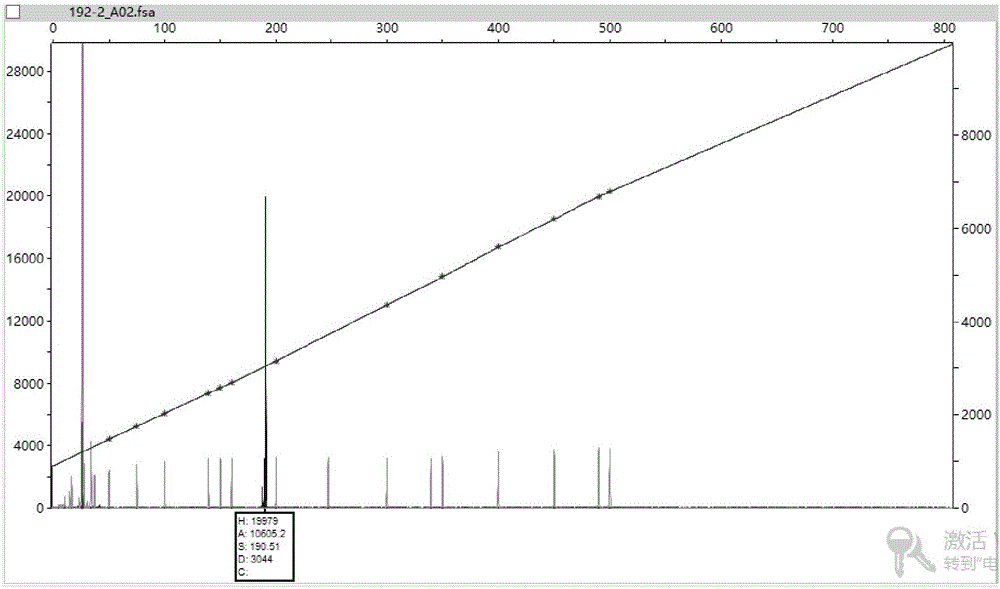
Image
Examples
Embodiment Construction
[0042] 1 Polymerase chain reaction (PCR) amplification of the target band
[0043] 1.1 PCR system (25ul)
[0044]
[0045] Note: ①5*PCR enhancer components: 2.7M betaine, 6.7mM DTT, 6.7% DMSO, and 55ug / ml BSA.
[0046] ②The PCR reaction system (except primers used) and temperature (below) for agarose gel electrophoresis, capillary electrophoresis and T vector cloning and sequencing for the detection of different subtypes are all the same.
[0047] ③Primer usage rules: Different primers are used for amplification between different subtypes, and the specific primer sequences are shown in SEQ ID NO. Primers, PCR for capillary electrophoresis must use fluorescent forward or reverse primers.
[0048] 1.2 PCR reaction temperature
[0049]
[0050] 2 agarose gel electrophoresis
[0051] 2.1 Reagents and materials
[0052]⑴ electrophoresis buffer (0.5×TBE or 1×TBE)
[0053] 5×TBE (stock solution): 0.45mol / L Tris base, 45mol / L boric acid, 0.01mol / L EDTA (stored at room temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com