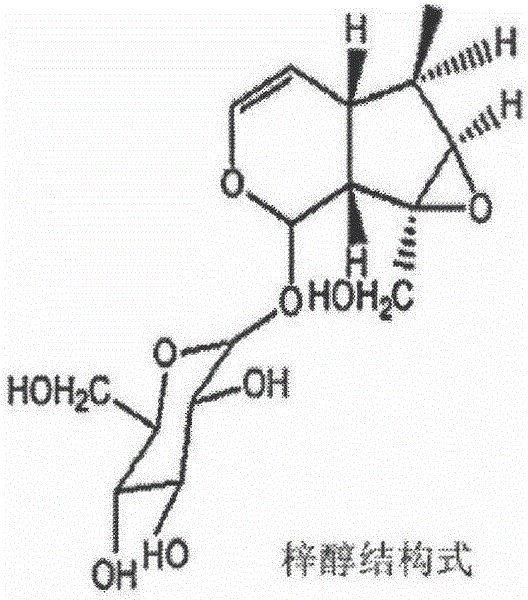
Application of catalpol in preparation of medicine for preventing and curing or delaying myasthenia and/or amyotrophia
A technology for muscle weakness and muscle atrophy, which is applied in drug combinations, pharmaceutical formulas, and muscular system diseases. It can solve the problems of diabetic myopathy with little curative effect and no cause and target of diabetic myopathy, so as to delay muscle weakness. and/or muscle atrophy, improvement of decreased exercise capacity, and improvement of muscle weakness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
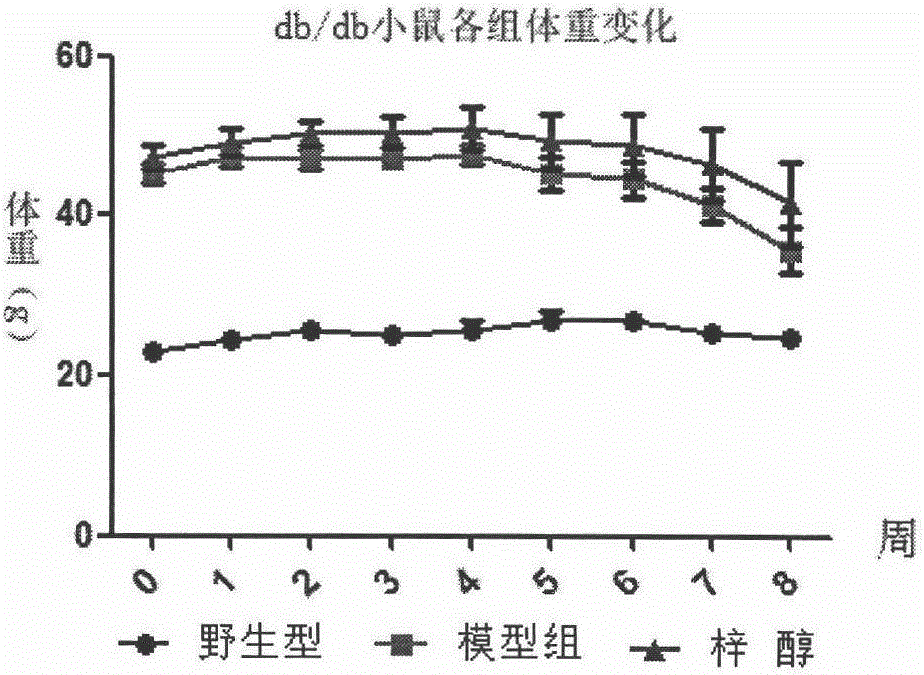
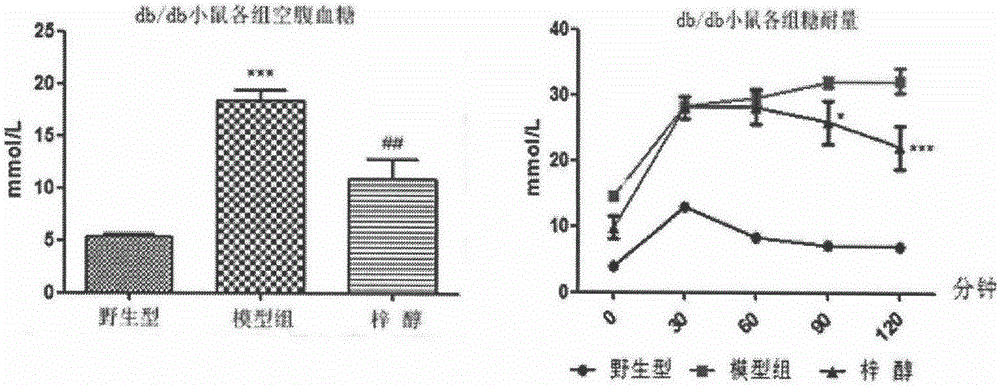
[0020] The pharmacodynamics evaluation of catalpol treating myasthenia caused by late stage diabetes mellitus, the specific method is as follows: buy 12 db / db (C57BL / KsJ) mice, 6 C57BL / 6NCrlVr mice, wild-type C57BL / 6NCrlVr mice as The normal control NC group (n=6), db / db (C57BL / KsJ) mice were randomly divided into two groups according to body weight stratification, named respectively as db / db model group (n=6) and catalpol treatment group (200mg / kg, n=6) The specific grouping and administration methods are as shown in Table 1:
[0021] Table 1. The specific grouping of experimental mice and the dosage and administration method
[0022] group number of animals Method of administration Dosage Time point of first administration (8 weeks old) NC group 6 Equal volume of distilled water 0.1ml / 10g 57d db / db model group 6 Equal volume of distilled water 0.1ml / 10g 57d catalpol treatment group 6 catalpol 200mg / kg 57d
[0023] Admi...
Embodiment 2
[0036] Catalpol treatment of HFD-induced early diabetes-induced pharmacodynamics evaluation, the specific method is as follows: 64 C57BL / 6NCrlVr mice were purchased, and the mice were randomly divided into 8 groups according to body weight, respectively NC control group 1 ( n=8), IFG model group (n=8), catalpol treatment group 1 (n=8), metformin treatment group 1 (n=8), NC control group 2 (n=8), IGT model group (n =8), catalpol treatment group 2 (n=8), metformin treatment group 2 (n=8). Except NC control group 1 and NC control group 2 were fed with normal feed RD (Nantong Trophy Feed Technology Co., Ltd.), and other groups were fed with high-fat feed HFD (TP24200, Nantong Trophy Feed Technology Co., Ltd.). After 6 weeks of HFD modeling, the success of the impaired fasting glucose regulation (IFG) model was verified. NC control group 1 (n=8), IFG model group (n=8), catalpol treatment group 1 (n=8), metformin treatment Group 1 (n=8) began to administer drugs, and each group was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com