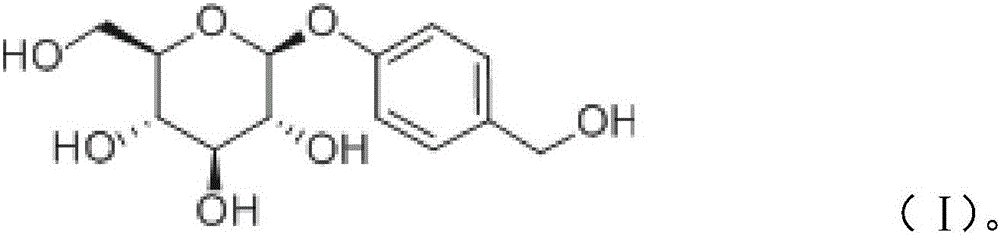
Gastrodin derivative, and preparation method, application and medicinal preparation of gastrodin derivative
A kind of pharmaceutical preparation, technology of gastrodin, applied in the field of gastrodin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The present invention also provides a preparation method of gastrodin derivatives, comprising:
[0045] Under the action of a catalyst, the compound having the structure of formula (XI) is reacted with the compound having the structure of formula (XII) in a solvent to obtain a gastrodin derivative having the structure of formula (II);
[0046]
[0047] R-OH (Ⅻ);
[0048]
[0049] Wherein, R is a substituted indene ring.
[0050] In the process of preparing gastrodin derivatives, the compound of formula (XI) is tetraacetylglucose trichloroacetimidate, which can be prepared according to the preparation methods well known to those skilled in the art, and this application has no special limits. The molar ratio of the tetraacetylglucose trichloroacetimidate to the compound having the structure of formula (XII) is preferably 2 to 3:1, the reaction temperature is preferably 20 to 30°C, and the reaction time is Preferably it is 1-3h. The reaction process of gastrodin ...
Embodiment 1
[0065] The preparation of embodiment 1KPC-4000006
[0066]
[0067] In a 25ml two-necked flask, add compound 1 (150mg, 1.0mmol, 1.0eq), compound 2 (748mg, 1.52mmol, 1.5eq) and molecular sieves (3.0g, 4A), use the vacuum oil pump to remove the air inside the reaction device Replaced with nitrogen, added dichloromethane (5.0ml) to the flask successively, placed the flask in an ice bath, and stirred for 30 minutes; slowly added boron trifluoride diethyl ether (0.15mL, 1.21mmol, 1.2eq ); continue stirring for 30 minutes after the dropwise addition, then remove the ice bath, allow the reaction solution to naturally warm up to room temperature (20°C), and use TLC to detect the reaction process until the raw material disappears (2 hours); slowly pour the reaction solution into a A beaker filled with crushed ice (20g) was quenched, and until the ice cubes melted completely, the aqueous phase of the resulting mixed solution was extracted with ethyl acetate (20ml×3); the organic phas...
Embodiment 2
[0069] The preparation of embodiment 2KPC-4000007
[0070]
[0071] In a 25ml two-necked flask, add compound 4 (150mg, 1.0mmol, 1.0eq), compound 2 (748mg, 1.52mmol, 1.5eq) and molecular sieves (3.0g, 4A), use the vacuum oil pump to remove the air inside the reaction device Replaced with nitrogen, added dichloromethane (5.0ml) to the flask successively, placed the flask in an ice bath, and stirred for 30 minutes; slowly added boron trifluoride diethyl ether (0.15mL, 1.21mmol, 1.2eq ), continue to stir for 30 minutes after the dropwise addition; then remove the ice bath, allow the reaction solution to naturally warm up to room temperature (20 ° C), and use TLC to detect the reaction process until the raw materials disappear (2 hours); slowly pour the reaction solution into a A beaker filled with crushed ice (20g) was quenched, and until the ice cubes melted completely, the aqueous phase of the resulting mixed solution was extracted with ethyl acetate (3×20ml), the organic pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com