A microfluidic chip and its application in pathogen identification and drug sensitivity test
A microfluidic chip and chip technology, applied in the field of pathogen identification and drug sensitivity experiment, can solve the problems of cumbersome operation, not conducive to timely diagnosis and antibiotic selection guidance, and unable to effectively meet clinical work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
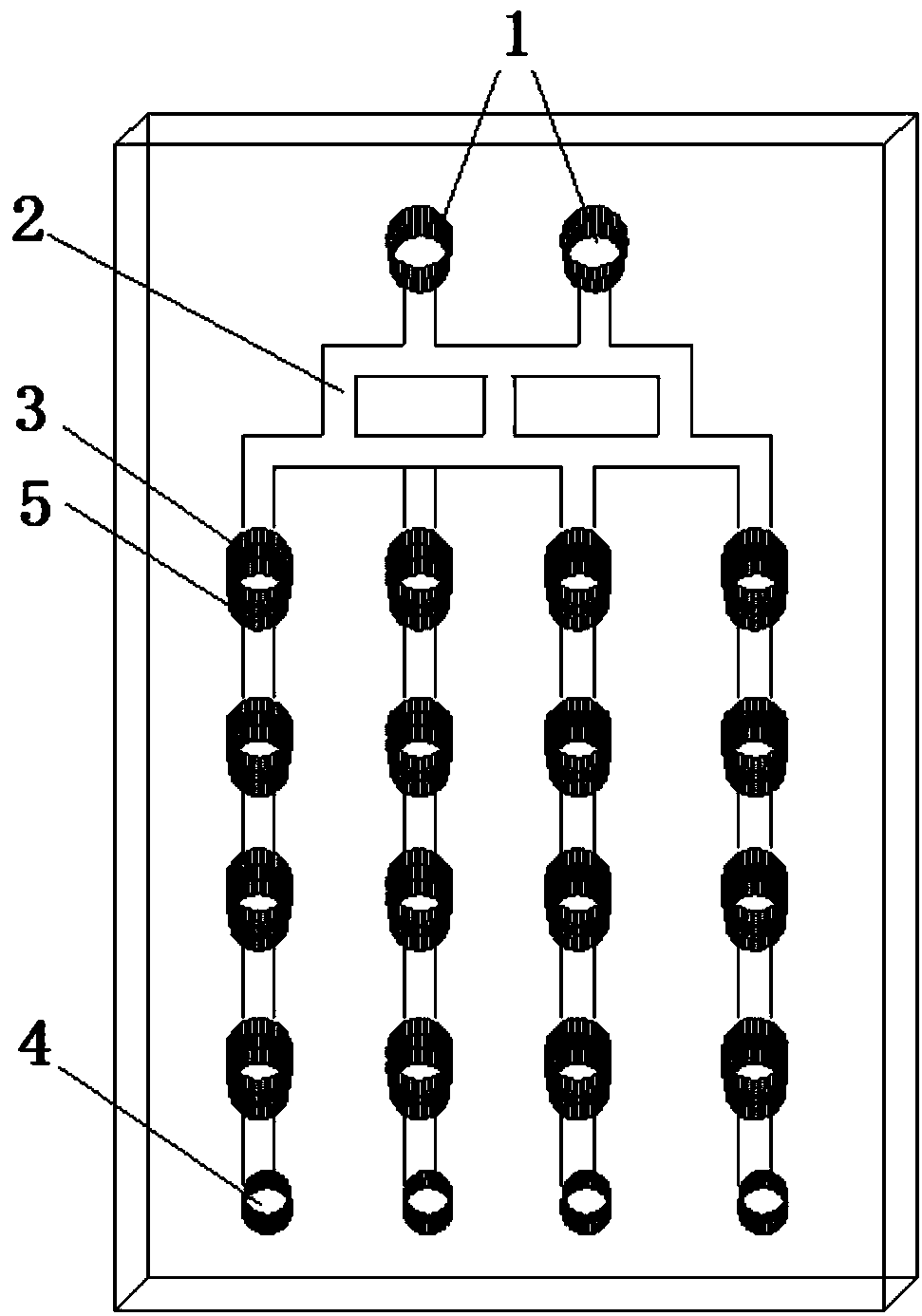
[0038] The microfluidic chip of the present invention comprises a 3-layer PDMS structure:
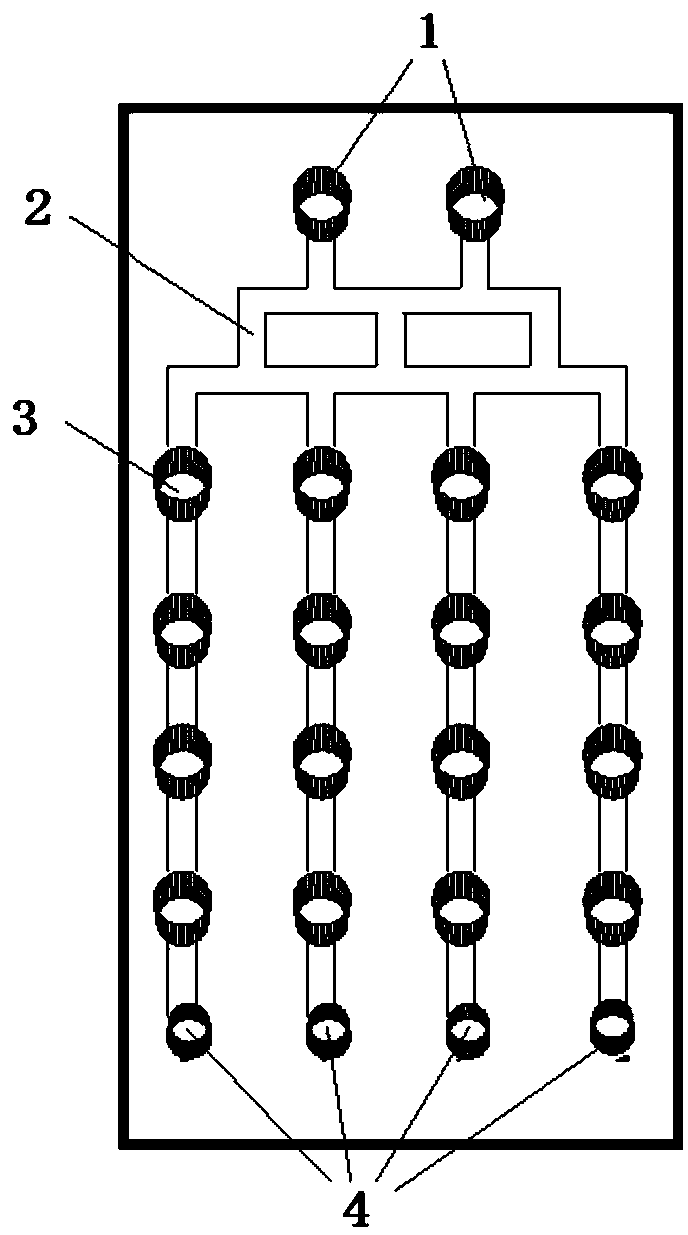
[0039] The top layer of the microfluidic chip has inlets, liquid channels, grooves, and waste holes; the middle layer of the microfluidic chip has a culture pool, and the bottom layer of the microfluidic chip is a flat structure, such as a glass slide; where:
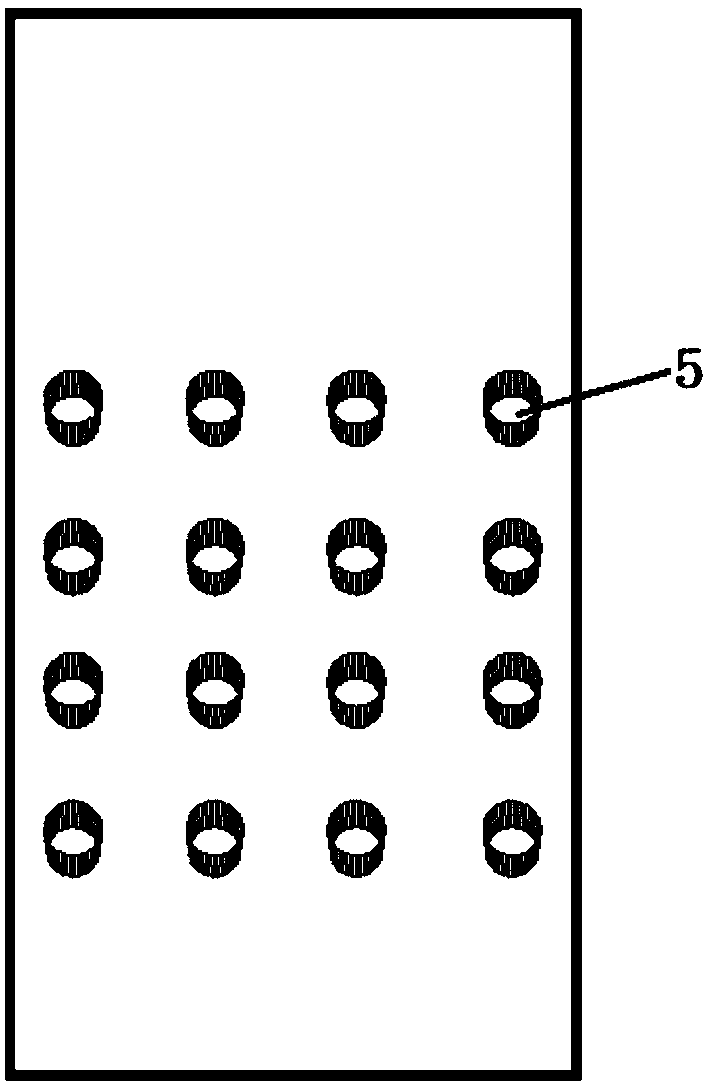
[0040] The culture pool in the middle layer of the microfluidic chip has 16 through holes arranged in a matrix of 4 columns×4 rows, the diameter of the culture pool is 3mm, and the height of the culture pool is 2mm.
[0041] There are two entrances on the top layer of the microfluidic chip; there are 16 grooves arranged in a matrix of 4 columns×4 rows, with a diameter of 3 mm. The liquid channel between the two inlets and the groove is called a concentration generator, and the number of channels in the longitudinal direction of the concentration generator is divided into 3 by 2 at the entrance, and then divided into 4 by 3, and f...
Embodiment 2
[0045] Chip sealing process:
[0046] Each layer of the chip was sterilized by autoclaving at 121°C for 30 minutes. Chip sealing is completed under sterile conditions. Firstly, the middle layer and the lower layer of the chip are subjected to plasma treatment respectively, and then sealed, and then a liquid specific chromogenic medium is added to the culture pool, and the liquid specific chromogenic culture medium is The base means that after the culture medium has been sterilized by high temperature and high pressure, it is in a liquid state (below about 45°C) before it is cooled; get the full chip. The sealed chip was stored in a humid box at 4°C, and the surface of the chip was irradiated with an ultraviolet lamp for 1 h before use.
[0047] Among them, the types of culture medium are respectively: the first line adds Candida albicans identification medium, the second line adds Escherichia coli identification medium, the third line adds Staphylococcus aureus identificatio...
Embodiment 3
[0049] Application of the microfluidic chip of the present invention in identification of cervical pathogens and drug sensitivity test
[0050] Before each experiment, inject absolute ethanol into the two inlets of the chip to fill the entire liquid channel, let it stand for 10 minutes, and then rinse the chip liquid channel 3 times with sterile distilled water.
[0051] The micro-syringe and the polytetrafluoroethylene capillary were soaked in 75% alcohol for 6 hours, rinsed with sterilized distilled water for 3 times, and irradiated with ultraviolet light for 30 minutes.
[0052] When in use, connect the microsyringe with the Teflon capillary on the ultra-clean bench. The hawthorn core extract was selected as the test drug for the drug susceptibility test, and the hawthorn core extract (25mg / mL) was pumped into the two inlets of the concentration generator, the right inlet, and the normal saline was pumped into the other hole, and samples were added to the two holes at the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com