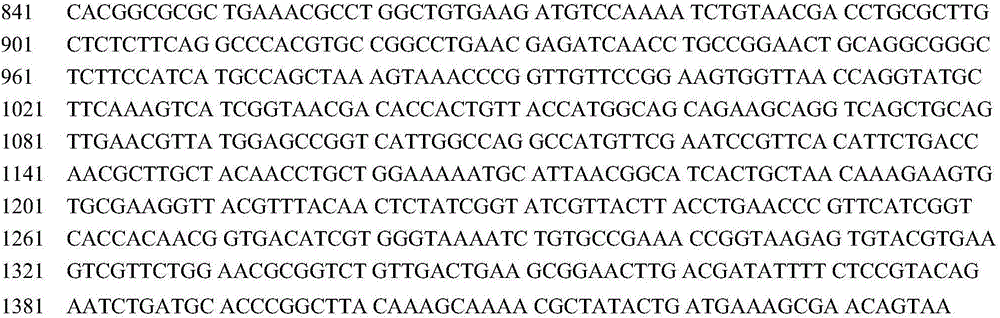Method for accelerating synthesis of L-arginine through enhancing ammonia assimilation
An assimilation, arginine technology, applied in microorganism-based methods, biochemical equipment and methods, nucleic acid carriers, etc., can solve problems such as no relevant research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Effects of individual expression of glutamate dehydrogenase, glutamine synthase and aspartase on L-arginine synthesis
[0025] 403 / pDXW-10-gdh, 403 / pDXW-10-glnA and 403 / pDXW-10-aspA were fermented in shake flasks, and the composition and fermentation conditions of the fermentation medium were the same as those in the control example. Fermentation 60h L-arginine yield 403 / pDXW-10-gdh was 15.7g / L, 403 / pDXW-10-glnA was 17.5g / L, 403 / pDXW-10-aspA was 18.1g / L.
Embodiment 2
[0026] Example 2: Effect of co-expression of glutamine synthase and aspartase on L-arginine synthesis
[0027] It can be seen from Example 1 that overexpression of glutamine synthase and aspartase alone can significantly increase the production of C. crenatumSDNN403 L-arginine, while overexpression of glutamate dehydrogenase L-arginine production does not increase significantly . This may be because the original glutamate dehydrogenase activity of C. crenatum SDNN403 can meet the needs of L-arginine synthesis. Therefore, the effect of co-expression of glutamine synthase and aspartase on L-arginine synthesis was continued. 403 / pDXW-10-glnA-aspA shake flask fermentation, the fermentation medium composition and fermentation conditions are the same as those in the control example. The yield of L-arginine was 19.3g / L after 60h fermentation.
Embodiment 3
[0028] Example 3: Effect of co-expression of glutamine synthase, aspartase and glutamate dehydrogenase on L-arginine synthesis
[0029] It can be seen from Example 2 that excessive co-expression of glutamine synthase and aspartase can further increase the L-arginine production of C. crenatum SDNN403. 403 / pDXW-10-glnA-aspA L-arginine synthesis ability was significantly improved, then L-glutamic acid was used as the direct precursor of L-arginine synthesis, and L-glutamic acid in strain 403 / pDXW-10-glnA-aspA Amino acid supply has the potential to be the limiting factor for L-arginine synthesis. Therefore, the effect of co-expression of glutamine synthase, aspartase and glutamate dehydrogenase on L-arginine synthesis was continued. 403 / pDXW-10-glnA-aspA-gdh shake flask fermentation, fermentation medium composition and fermentation conditions are the same as the control example. The L-arginine yield was 20.8g / L after 60h fermentation.
[0030]
[0031]
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com