A photoresponsive methyl methacrylate azo polymer and its synthesis method
A technology of methyl methacrylate and methyl methacrylate, which is applied in the field of polymer synthesis and preparation, and achieves the effects of high yield, mild reaction conditions and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
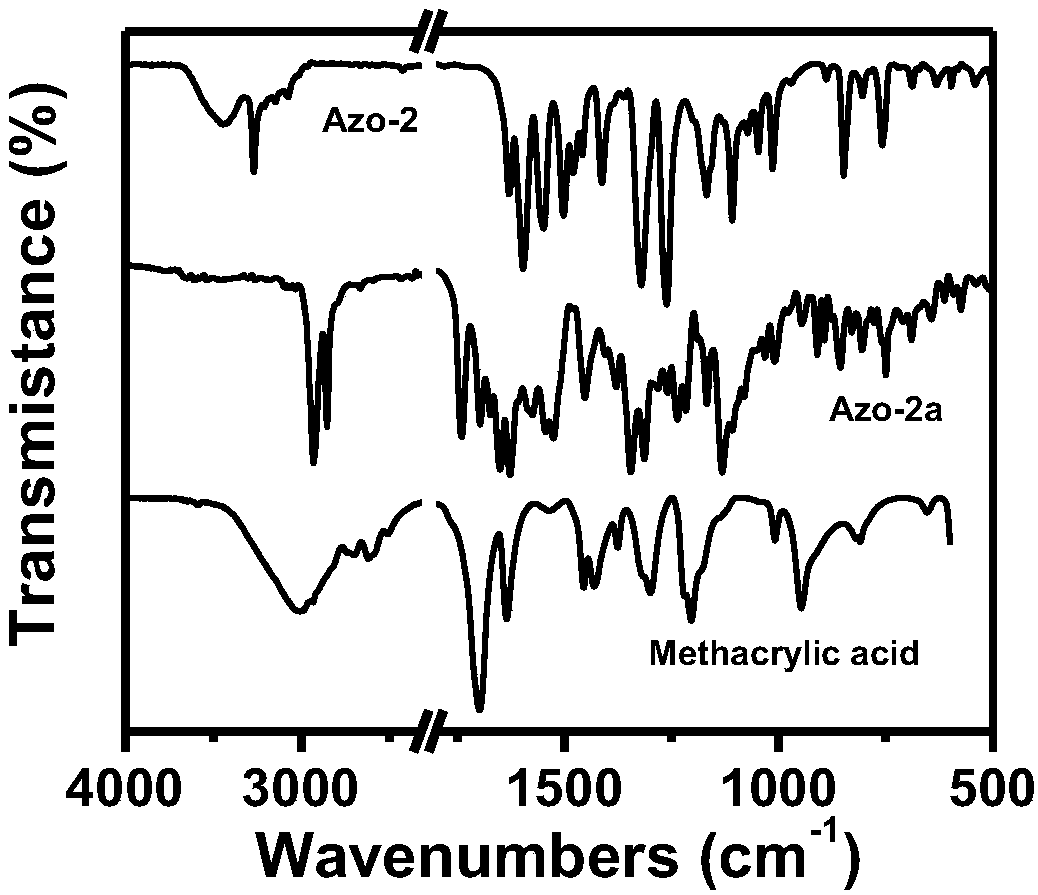
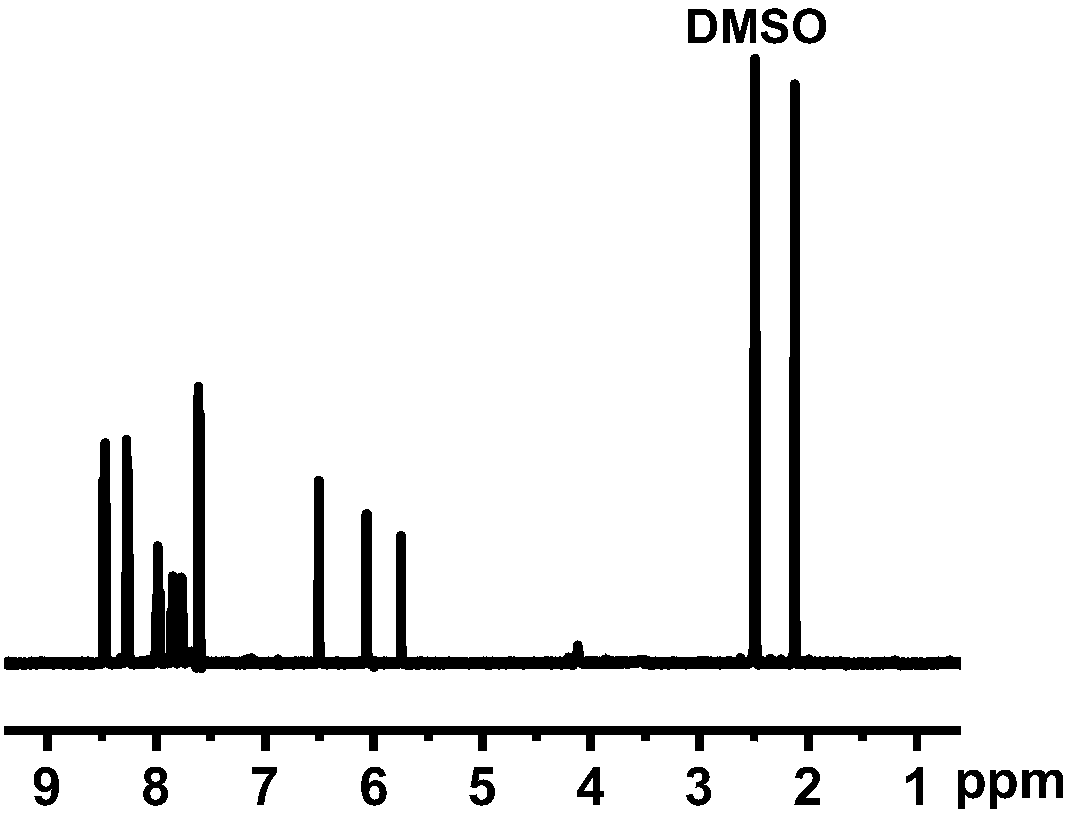
[0035] (1) Synthesis of azo monomer: at 0°C, with dichloromethane as solvent, slowly drop 0.01mol / L 4-(4-nitrophenylazo)-1-naphthol solution into toluene In base acrylic acid, the molar mass ratio of 4-(4-nitrophenylazo)-1-naphthol to methacrylic acid is 2:1; add 4-dimethylaminopyridine (DMAP) and dicyclohexyl carbon to the system Diimine (DCC), the molar mass ratio of DMAP and DCC is 1:5. First react at 0°C for 2 hours, then raise the temperature to 25°C, and continue the reaction for 12 hours to obtain the azo monomer. The structural formula is:
[0036]
[0037] (2) Synthesis of methyl methacrylate-based azo polymers: carry out polycondensation reaction between methyl methacrylate and the synthesized azo monomer in a molar mass ratio of 1:10, and the addition amount of the initiator AIBN is 1% of the amount of methyl acrylate added. First add methyl methacrylate and AIBN into the container, blow nitrogen at 25°C to remove oxygen in the system; then slowly add the azo ...
Embodiment 2
[0039] (1) Synthesis of azo monomer: at 0°C, with dichloromethane as solvent, slowly drop 0.1mol / L 4-(4-nitrophenylazo)-1-naphthol solution into toluene In the base acrylic acid, the molar mass ratio of 4-(4-nitrophenylazo)-1-naphthol to methacrylic acid is 1:1. Add 4-dimethylaminopyridine (DMAP) and dicyclohexylcarbodiimide (DCC) to the system, the molar mass ratio of DMAP and DCC is 1:10; first react at 0°C for 2 hours, then heat up to 25°C , continue to react for 12 hours to obtain azo monomer. The structural formula is:
[0040]
[0041] (2) Synthesis of methyl methacrylate azo polymer: methyl methacrylate and the azo monomer synthesized in step 1 are subjected to polycondensation reaction in a ratio of 1:15 by molar mass ratio, the addition amount of initiator AIBN 3% of the amount of methyl methacrylate added. First, add methyl methacrylate and AIBN into the container, blow nitrogen at 25°C to remove oxygen in the system; then slowly add the azo monomer prepared in...
Embodiment 3
[0048] (1) Synthesis of azo monomer: at 0°C, with dichloromethane as solvent, slowly drop 1mol / L 4-(4-nitrophenylazo)-1-naphthol solution into methyl In acrylic acid, the molar mass ratio of 4-(4-nitrophenylazo)-1-naphthol to methacrylic acid is 1:5. Add 4-dimethylaminopyridine (DMAP) and dicyclohexylcarbodiimide (DCC) to the system, the molar mass ratio of DMAP and DCC is 1:20; first react at 0°C for 5 hours, then heat up to 25°C , continue to react for 24 hours to obtain azo monomer. The structural formula is:
[0049]
[0050] (2) Synthesis of methyl methacrylate azo polymer: carry out polycondensation reaction with methyl methacrylate and the azo monomer synthesized in step 1 in a molar mass ratio of 1:100, the addition amount of initiator AIBN 5% of the amount of methyl methacrylate added. First add methyl methacrylate and AIBN into the container, blow nitrogen at 25°C to remove oxygen in the system; then slowly add the azo monomer prepared in step 1 to the system d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com