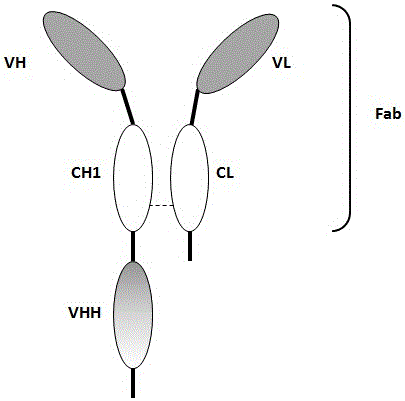
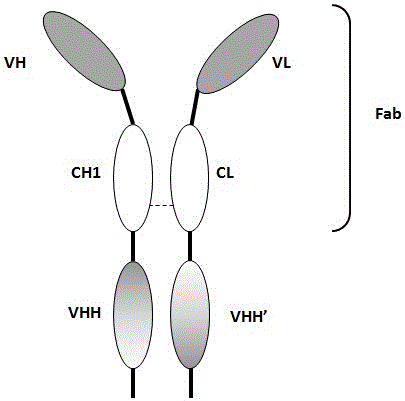
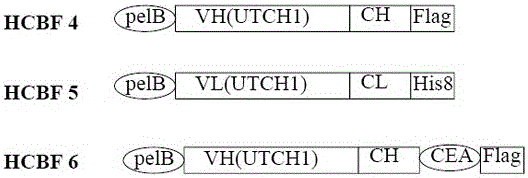
Bivalent antibody having single-domain antigen-binding fragment fused to conventional Fab fragment
A bivalent antibody and binding fragment technology, applied in the direction of antibodies, anti-tumor drugs, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Example 1. Bispecific antibodies induce T-cell-mediated cytotoxicity
[0151] This example provides a novel bispecific antibody format by linking a single-domain anti-CEA VHH targeting CEA-positive tumor cells to an anti-CD3 Fab that binds T cells. Such an antibody (S-Fab) can recruit T cells to CEA-positive tumor cells. By involving T cells, this S-Fab antibody effectively kills CEA-positive tumor cells in vitro. In xenograft models, the S-Fab antibody exhibited potent tumor suppressive effects, suggesting that such a strategy could be used in many immunotherapies. Furthermore, the S-Fab antibody can be efficiently and stably expressed and purified from E.coli.
[0152] This example utilizes the combination of single domain antibodies (VHH) and Fab fragments to produce heterodimeric antibodies. In this format, an anti-CEA VHH is linked to the C-terminus of a partial heavy chain of an anti-CD3 antibody (VH-CH1), and the resulting VH-CH1-VHH chain is combined with an ...
Embodiment 2
[0191] Example 2. Bispecific antibodies induce NK-cell-mediated cytotoxicity
[0192] This example provides a new bispecific antibody form Her2-S-Fab, by linking single domain anti-CD16 VHH to trastuzumab (Trastuzumab, ) constructed from the C-terminus of the Fab. Her2-S-Fab can be expressed and purified by bacterial cells. In vitro cell experiments, Her2-S-Fab can specifically kill Her2 overexpressed cancer cells by recruiting NK cells. Enhanced tumor cell killing was observed compared with trastuzumab. In vivo studies have shown that Her-2-S-Fab can inhibit tumor progression.
[0193] Methods and Materials
[0194] Fab design and protein purification
[0195] The constructs of Her2-S-Fab and control Her2-Fab are shown in Fig. 6a. By standard DNA cloning techniques, first, trastuzumab anti-Her2 VL-CL and VH-CH1 (see Table 5) were chemically synthesized and cloned into pET21a vector and pET26b vector. Subsequently, VHH-CD16 was cloned into the C-terminus of trastuzumab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com