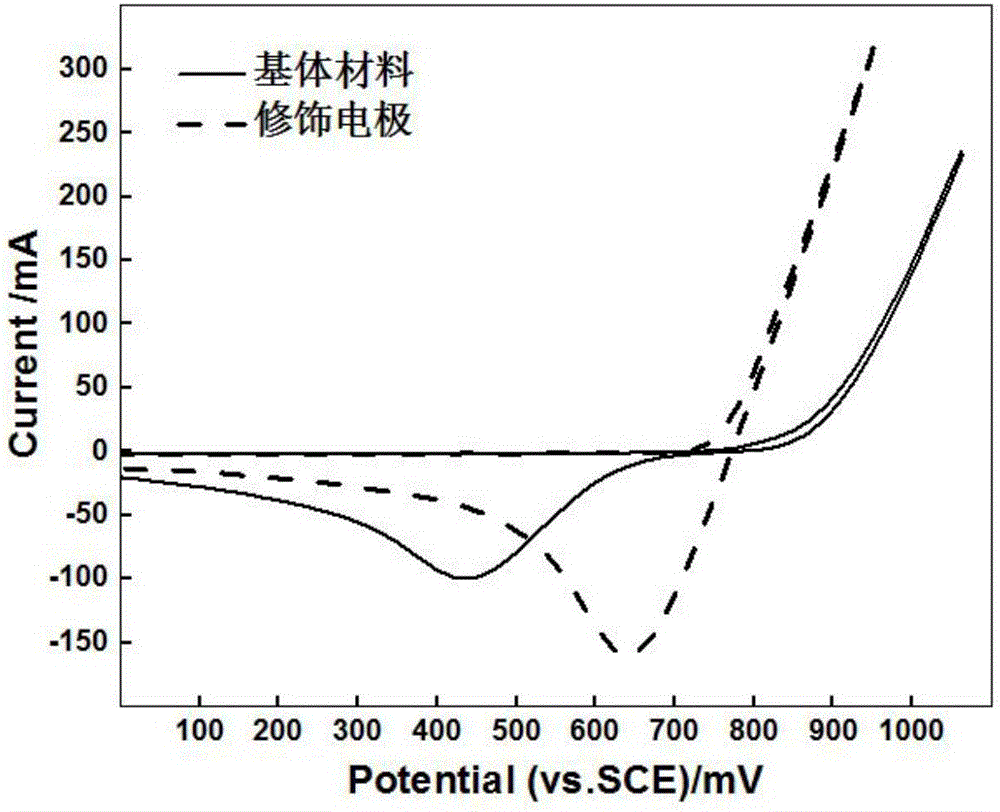
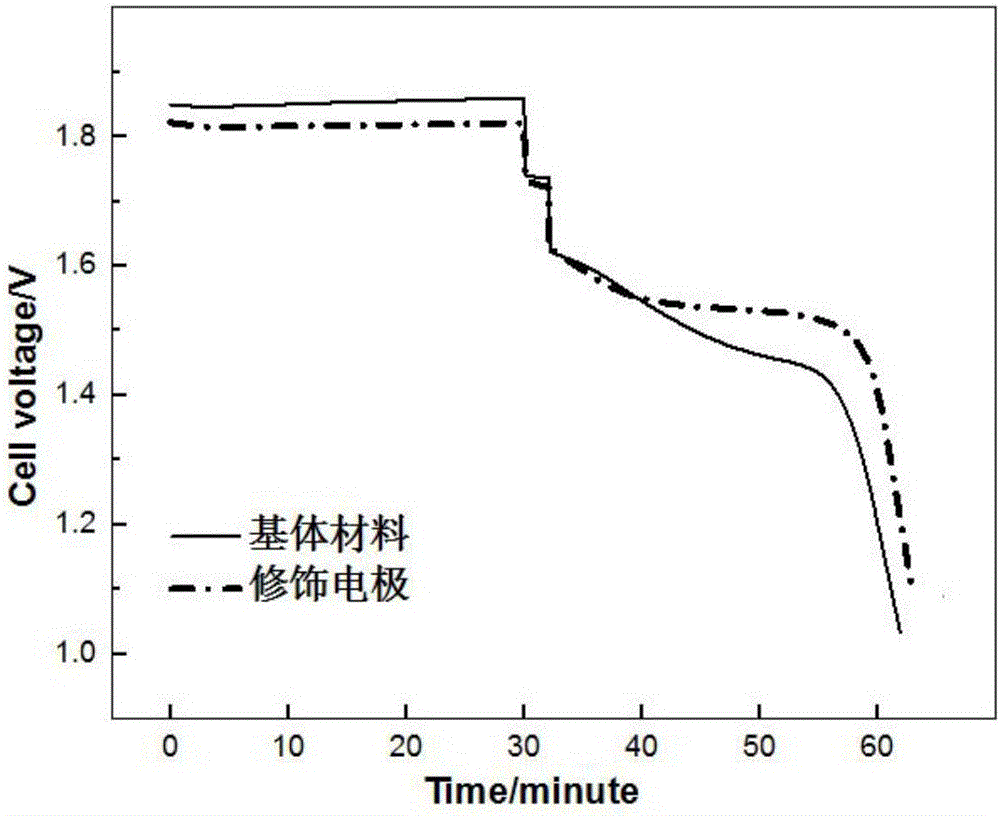
Modified electrode applied to zinc-bromine redox flow battery and preparation method thereof
A technology for zinc-bromine flow batteries and modified electrodes, which can be used in battery electrodes, fuel cells, regenerative fuel cells, etc., and can solve problems such as cost increase and multiple single cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
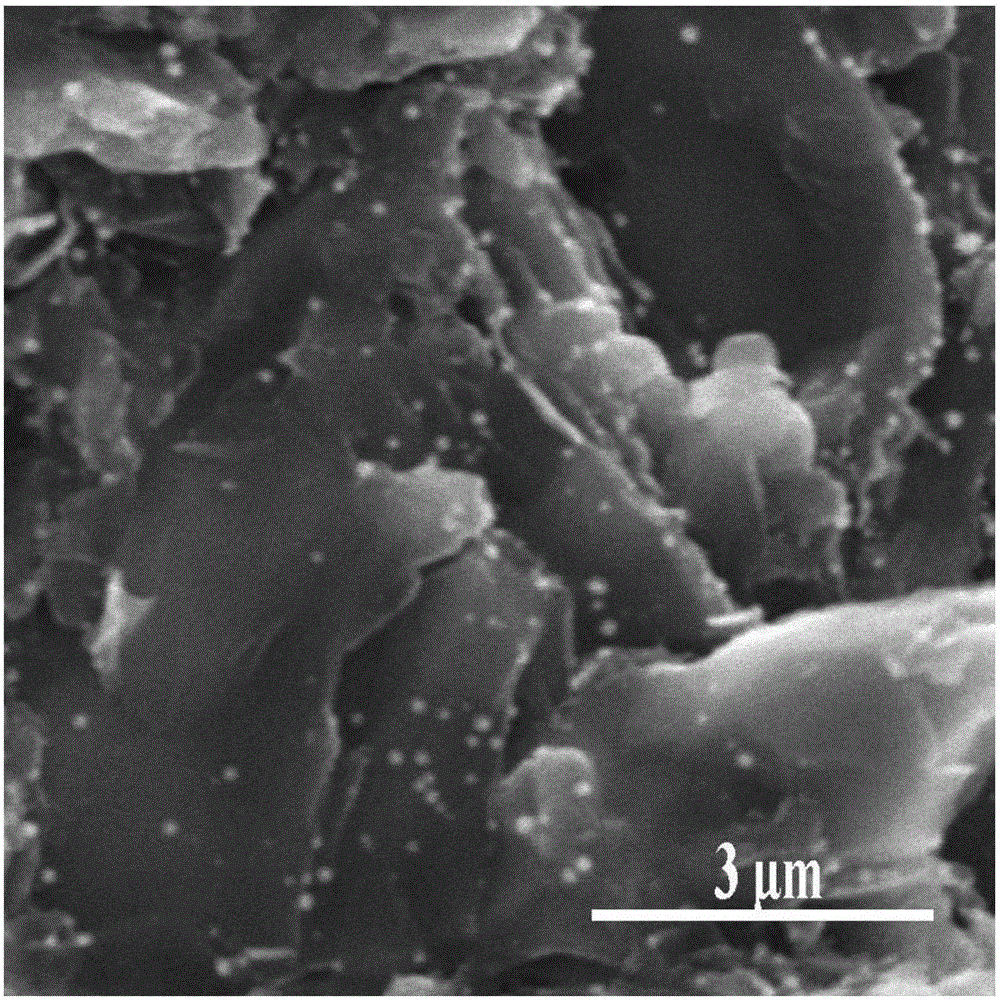
[0016] Weigh 32.37 mg of chloroplatinic acid hexahydrate into 25 ml of deionized water, and oscillate ultrasonically for 2 minutes to mix the precursor solution evenly. Then take a 1cm×5cm graphite sheet, polish it with 60cw sandpaper to make the surface rough, rinse it with deionized water, and dry it. Immerse the treated graphite sheet in the precursor solution and let it stand for 20 minutes to make the precursor solution fully contact with the surface of the graphite sheet. Then, prepare 25ml of 0.0025M ascorbic acid solution, add the ascorbic acid solution drop by drop into the precursor solution soaked in graphite flakes with a rubber dropper, stop adding the reducing agent solution and let it stand for 30 minutes to make the reducing agent and the precursor solution fully reaction. The graphite sheet was then taken out and dried in vacuum at 40° C. for 12 hours to complete the preparation of a modified electrode with platinum nanoparticles on the surface.
Embodiment 2
[0018] Weigh 22.20 mg of ammonium chloropalladate in 25 ml of deionized water and oscillate ultrasonically for 2 min to mix the precursor solution evenly. Then take a 1cm×5cm graphite sheet, polish it with 60cw sandpaper to make the surface rough, rinse it with deionized water, and dry it. Immerse the treated graphite sheet in the precursor solution and let it stand for 20 minutes to make the precursor solution fully contact with the surface of the graphite sheet. Then, take 7ml of 85% hydrazine hydrate solution, add the hydrazine hydrate solution dropwise into the precursor solution soaked in graphite flakes with a rubber dropper, stop adding the reducing agent solution and let it stand for 30 minutes to make the reducing agent and the precursor The solution is fully reacted. The graphite sheet was then taken out and dried in vacuum at 40° C. for 12 hours to complete the preparation of a modified electrode with palladium nanoparticles on the surface.
Embodiment 3
[0020] Weigh 25.74 mg of chloroauric acid tetrahydrate into 25 ml of deionized water, and oscillate ultrasonically for 2 minutes to mix the precursor solution evenly. Then take a 1 cm × 5 cm carbon felt, rinse it with deionized water, and dry it under vacuum at 40 degrees Celsius. Dip the carbon felt into the precursor solution and let it stand for 20 minutes to make the precursor solution fully contact with the surface of the carbon felt fiber. Then, prepare 25ml of 0.0025M ascorbic acid solution, add the ascorbic acid solution drop by drop into the precursor solution soaked in carbon felt with a rubber dropper, stop adding the reducing agent solution and let it stand for 30 minutes to make the reducing agent and the precursor solution fully reaction. The carbon felt was then taken out, and dried under vacuum at 40° C. for 12 hours to complete the preparation of a modified electrode with gold nanoparticles modified on its surface.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com