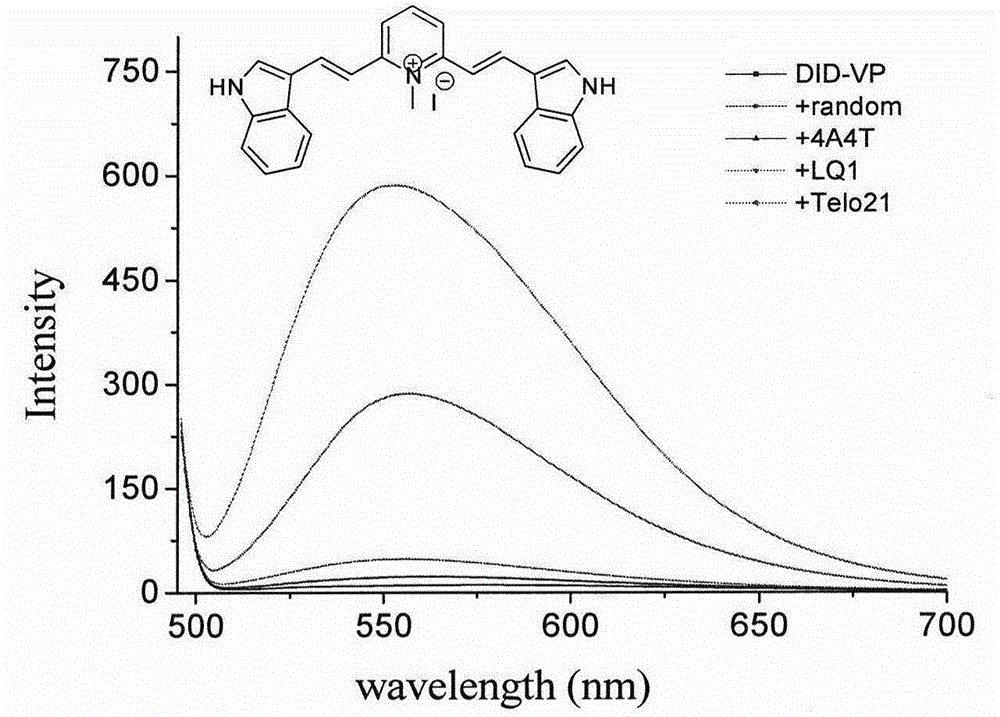
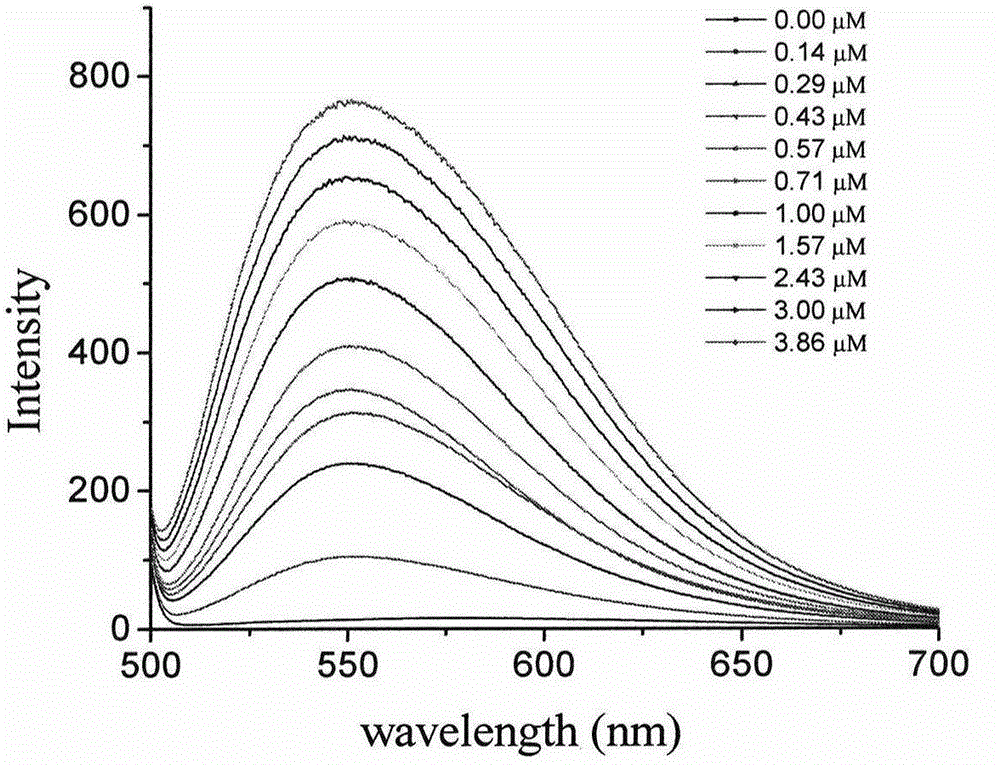
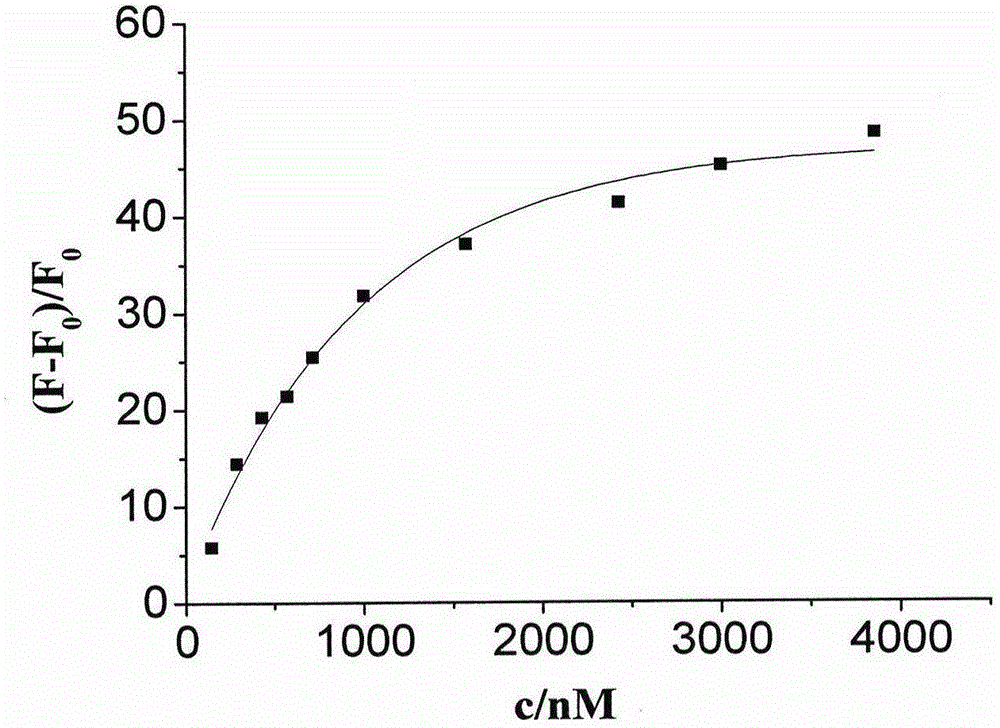
Fluorescent probe taking styrene polyperoxide substitutive pyridine compound as G-quadruplex nucleic acid
A fluorescent probe and quadruplex technology, applied in fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., can solve the problems of poor selectivity limiting the application of C61, failure to recognize the secondary structure of G-quadruplex, etc., and achieve a good cell membrane Permeability, good photostability, simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment one: the synthesis of compound 3c
[0037] Weigh 1.10 g of 2,6-lutidine, 5.00 g of sulfolane, and 2.50 g of methyl iodide with a balance in a fume hood, place them in a 50 mL round bottom flask and add a stirrer, place in an oil bath at 50°C, and turn on magnetic stirring. There was no obvious change in the reaction system and no reflux. After 4h, the reaction was stopped, and there was a white solid in the round bottom flask. After cooling to room temperature, 15 mL of ethyl acetate was added, and a white solid precipitated out. Continue magnetic stirring for 0.5 h, filter with suction, and wash the filter residue with ethyl acetate. After drying, it was weighed to obtain 2.44 g of 1,2,6-trimethylpyridinium iodide M-DP, with a yield of 95.31%. The product is white solid particles. Then weigh 0.51g of M-DP, 1.00g of indole-3-carboxaldehyde ID, 15mL of n-butanol, and 15 drops of 4-methylpiperidine, and place them in a 50mL round-bottomed flask successively...
Embodiment 2
[0038] Embodiment two: the synthesis of compound 6a
[0039]Weigh 1.10g of 2,4,6-collidine, 5.00g of sulfolane, and 2.20g of methyl iodide in a fume hood, place them in a 50mL round bottom flask and add a stirrer, put them in a 70°C oil bath, turn on the condensed water and Magnetic stirring. After 2h, the reaction stopped, there was a white solid in the round bottom flask, and the solution was pale yellow-green. After cooling to room temperature, 15 mL of ethyl acetate was added to the flask, and a white solid precipitated out. Stir magnetically for 0.5 h, filter with suction, and rinse the filter residue with an appropriate amount of ethyl acetate. The filter residue was dried and weighed to obtain 2.25 g of 1,2,4,6-tetramethylpyridinium iodide M-TP. Yield 85.50%. Then weigh 0.54g of M-TP, 1.66g of 4-methylthiobenzaldehyde, 15mL of n-butanol, and 15 drops of 4-methylpiperidine, and place them in a 50mL round-bottomed flask successively, in an oil bath at 95°C, and turn o...
Embodiment 3
[0040] Embodiment three: the synthesis of compound 6b
[0041] Weigh 1.10g of 2,4,6-collidine, 5.00g of sulfolane, and 2.20g of methyl iodide in a fume hood, place them in a 50mL round bottom flask and add a stirrer, put them in a 70°C oil bath, turn on the condensed water and Magnetic stirring. After 2h, the reaction stopped, there was a white solid in the round bottom flask, and the solution was pale yellow-green. After cooling to room temperature, 15 mL of ethyl acetate was added to the flask, and a white solid precipitated out. Stir magnetically for 0.5 h, filter with suction, and rinse the filter residue with an appropriate amount of ethyl acetate. The filter residue was dried and weighed to obtain 2.25 g of 1,2,4,6-tetramethylpyridinium iodide M-TP. Yield 85.50%. Then weigh 0.54g of M-TP, 1.45g of indole-3-carboxaldehyde, 15mL of n-butanol, and 15 drops of 4-methylpiperidine, and place them in a 50mL round-bottomed flask successively, in an oil bath at 110°C, turn on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com