Novel process for preparing prucalopride intermediate
A technology of reaction time and compound, applied in the direction of organic chemistry, etc., can solve the problems of stability and safety restriction of hypervalent iodine compounds, not meeting the quality requirements of prucalopride raw materials, low chemical purity, etc. The effect of heavy metal residues and high chemical purity of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of Methyl 4-piperidinecarboxylate Hydrochloride
[0042]
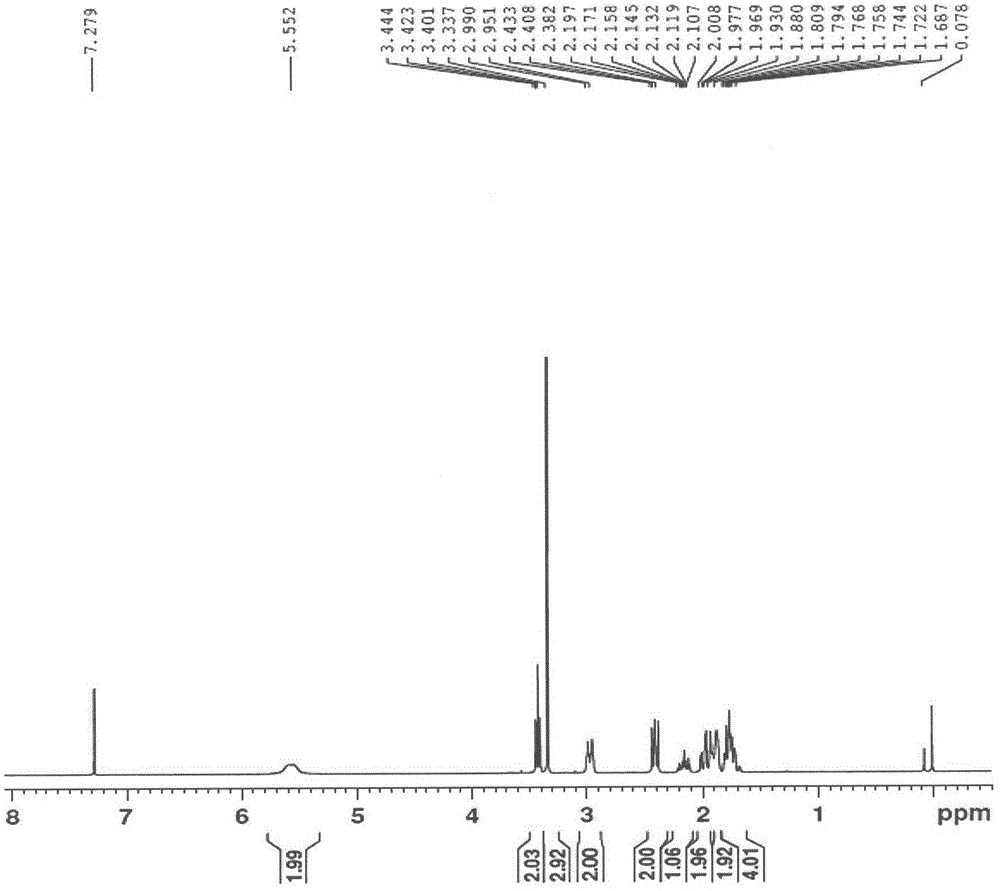
[0043] 4-piperidinecarboxylic acid (500g, 3.87mol) and methanol (2.5L) were added to the reaction flask, and thionyl chloride (460.4g, 3.87mol) was added dropwise. The temperature was raised to 80°C and the reaction was refluxed for 3h. The reaction solution was concentrated under reduced pressure at 50°C to obtain 687 g of white solid with a yield of 98.8%, which could be directly used in the next reaction. 1 H NMR (300MHz, CDCl 3 ) δ9.63 (m, 2H), 3.70 (s, 3H), 3.36 (m, 2H), 3.05 (m, 2H), 2.59 (m, 1H), 2.16 (m, 4H).
Embodiment 2
[0045] Synthesis of ethyl 4-piperidinecarboxylate hydrochloride
[0046]
[0047] The specific embodiment is the same as Example 1, and the yield is 97%.
Embodiment 3
[0049] Synthesis of 4-methylformate-1-(3-methoxypropyl)-piperidine (II-1)
[0050]
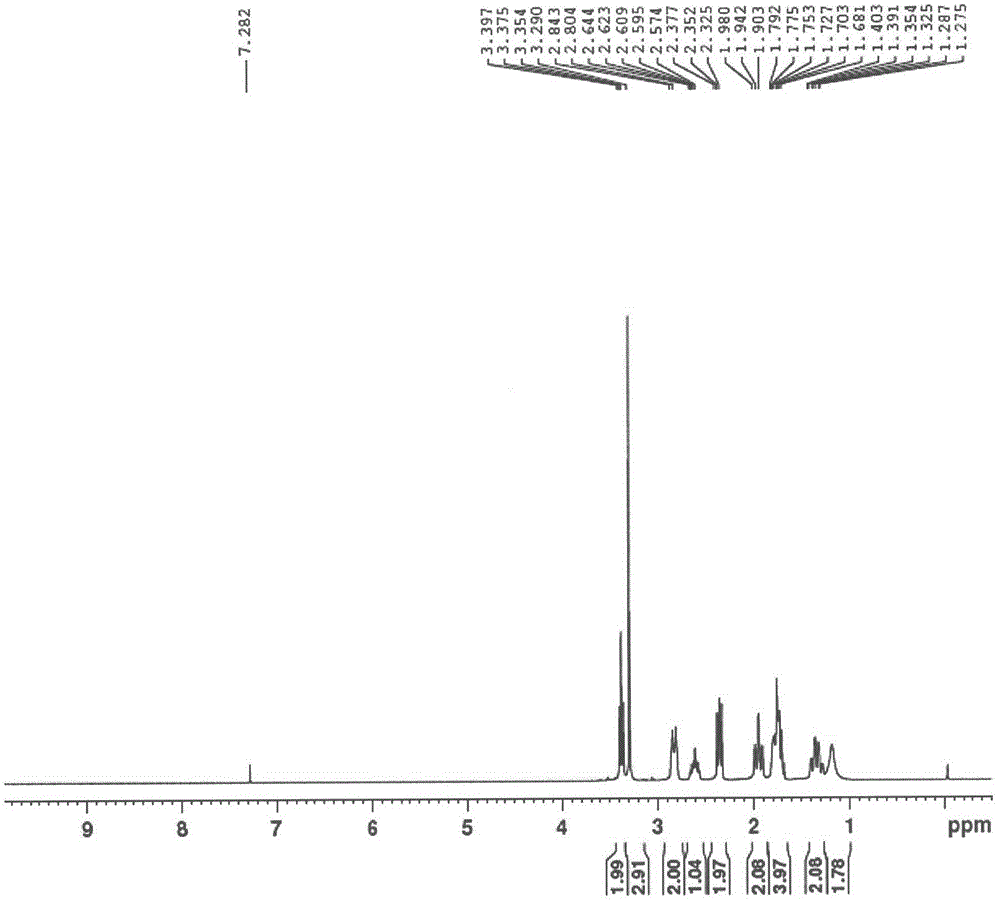
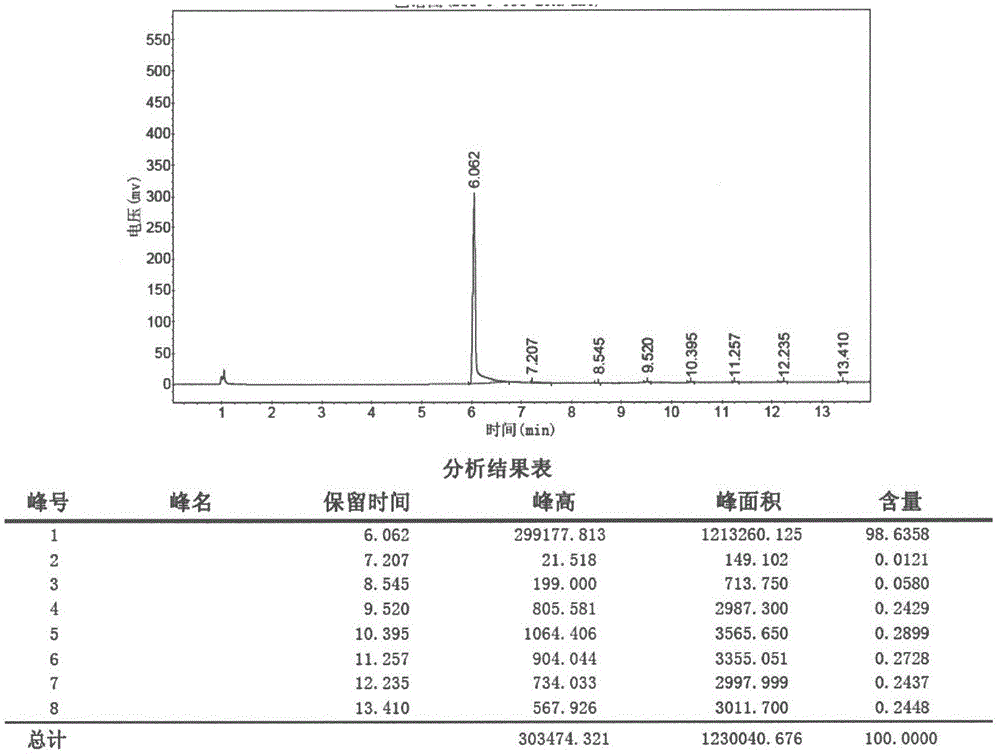
[0051]Methyl 4-piperidinecarboxylate hydrochloride (681.5g, 3.79mol), methanol (3.4L), potassium carbonate (1.3Kg, 9.48mol) and 3-bromopropyl methyl ether (638.4g, 4.17mol) They were added to the reaction flask in turn, and the external temperature was raised to 75°C and refluxed overnight. The reaction solution was filtered with suction, the filtrate was concentrated under reduced pressure at 60°C, the residue was added to 1.5 L of water, extracted with DCM, the organic layers were combined and dried over anhydrous sodium sulfate, and the organic layer was concentrated under reduced pressure at 60°C to obtain 590 g of a light yellow solid, with a yield of 72.2 %, can be directly used in the next reaction. 1 H NMR (300MHz, CDCl 3 )δ: 3.68(s, 3H), 3.39(t, J=6.3Hz, 2H), 3.31(s, 3H), 2.86(m, 2H), 2.24~2.42(m, 3H), 1.70~2.03(m , 8H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com