Dispersible tablet containing letrozole and preparation method thereof
A technology of letrozole and dispersible tablets, which is applied in the field of technology and preparation of solid preparation dispersible tablets, can solve the problems of large loss of raw materials, inability to completely solve the rapid dissolution and absorption of letrozole, and unsuitability for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
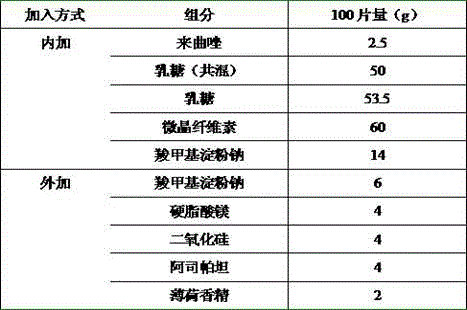
[0013]
[0014] Preparation Process:
[0015] Letrozole was crushed through a 200-mesh sieve, then mixed with 10 times the weight of lactose through an 80-mesh sieve for 3 times, added the remaining lactose, microcrystalline cellulose and sodium carboxymethyl starch, passed through a 80-mesh sieve for 3 times and mixed evenly, 2% hydroxypropyl Base cellulose as a binder, granulate with a 24 mesh sieve, blow dry at 50 ℃, dry to 1~3% moisture, pass the dried granules through a 24 mesh sieve for granulation, weigh, convert the yield, and add carboxymethyl Starch sodium, silicon dioxide, aspartame, mint flavor and magnesium stearate, Φ8 flat punched tablets, tablet weight 200 mg, hardness 20-40 N.
Embodiment 2
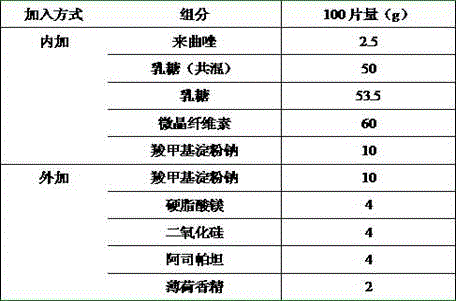
[0017]
[0018] Preparation Process:
[0019] Letrozole was crushed through a 200-mesh sieve, then mixed with 10 times the weight of mannitol through an 80-mesh sieve for 3 times, and the remaining mannitol, microcrystalline cellulose and sodium carboxymethyl starch were mixed through an 80-mesh sieve for 3 times, 2% Hydroxypropyl cellulose is used as a binder, granulated with a 24-mesh sieve, blast-dried at 50 °C, and dried to a moisture content of 1-3%, and the dried granules are passed through a 24-mesh sieve for granulation, weighed, converted yield, and carboxylated Sodium methyl starch, silicon dioxide, aspartame, mint flavor and magnesium stearate, Φ8 flat punched tablets, tablet weight 200 mg, hardness 20-40 N.
Embodiment 3
[0021]
[0022] Preparation Process:
[0023] Letrozole was crushed through a 200-mesh sieve, then mixed with 10 times the weight of lactose through an 80-mesh sieve for 5 times, and the remaining lactose, microcrystalline cellulose and sodium carboxymethyl starch were mixed through an 80-mesh sieve for 3 times, and 2% hydroxypropyl Base cellulose as a binder, granulate with a 24 mesh sieve, blow dry at 50 ℃, dry to 1~3% moisture, pass the dried granules through a 24 mesh sieve for granulation, weigh, convert the yield, and add carboxymethyl Starch sodium, silicon dioxide, aspartame, mint flavor and magnesium stearate, Φ8 flat punched tablets, tablet weight 200 mg, hardness 20-40 N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet weight | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com