Methods for diagnosing and treating inflammatory bowel disease
A gastroenteritis, reactive technique used in the field of diagnosis and treatment of inflammatory bowel disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0334] Phase II, Randomized, Double-Blind, Placebo-Controlled Study and Open-Label Extension Study to Evaluate the Efficacy and Safety of rhuMAbβ7 (etrolizumab) in Patients with Moderate-to-Severe Ulcerative Colitis
[0335] Clinical Study Description
[0336] rhuMAbβ7 (etrolizumab) Description
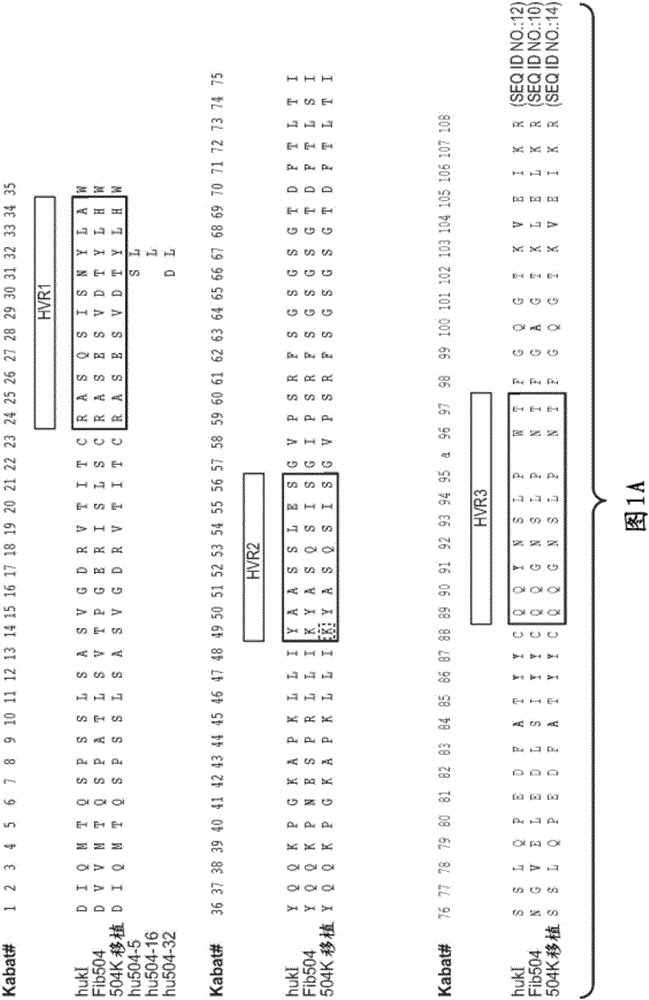
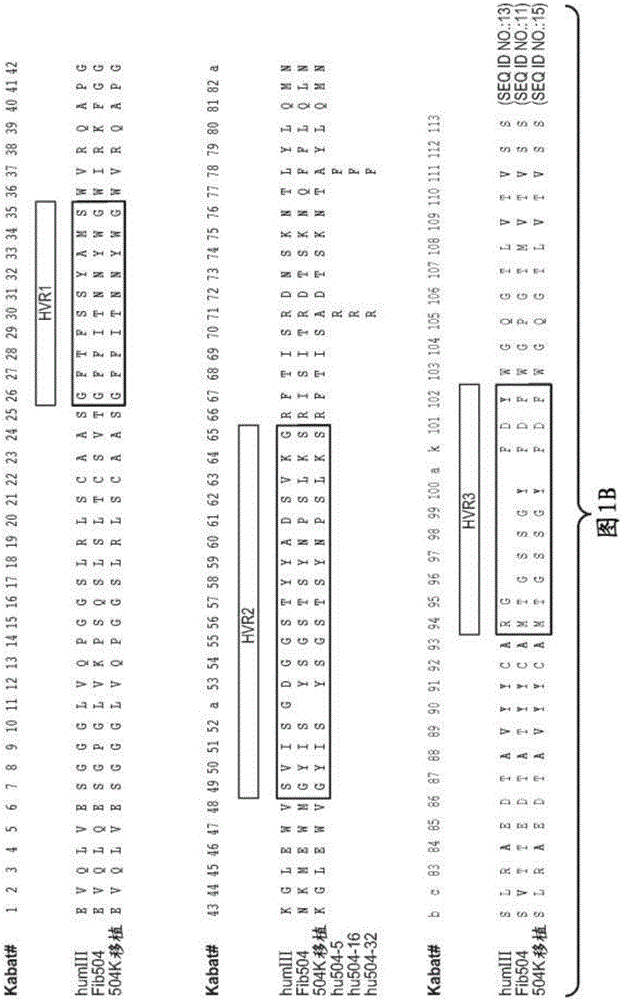
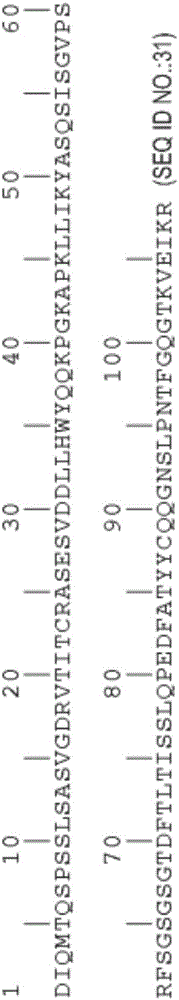
[0337] rhuMAbβ7 (etrolizumab) is a humanized monoclonal antibody based on human IgG1 subclass III V H , κ subtype-IV L Consensus sequence, and specificity against the β7 subunit of integrin heterodimers. see Figure 1A and B. It has been shown to bind α4β7 with high affinity (K of about 116 pM d ) and αEβ7 (K about 1800pM d ).
[0338] This recombinant antibody has two heavy chains (446 residues) and two light chains (214 residues) covalently linked by interchain and intrachain disulfide bonds typical of IgGl antibodies. For the studies described here, it was produced in Chinese Hamster Ovary (CHO) cells. The molecular weight of the intact aglycosylated rhuMAbβ7 molecule is a...
Embodiment 2
[0367] Example 2 - Predictive Biomarker Research and Analysis
[0368] Experimental design selected for enrichment of predictive biomarkers of efficacy in etrolizumab-treated patients
[0369] To identify novel biomarkers predictive of response to etrolizumab treatment, we first used RNA-sequencing to establish baseline gene expression profiles of colonic biopsies from all etrolizumab-treated patients. Baseline biopsies from etrolizumab-treated TNF antagonist-naïve patients were used to identify differential gene expression profiles between etrolizumab-treated patients who experienced remission and etrolizumab-treated patients who did not. Differentially expressed genes were further selected based on signal strength, biological relevance, and expression in other IBD datasets, and then further evaluated using quantitative polymerase chain reaction in colon biopsies from etrolizumab-treated patients and placebo-treated patients for subgroup analysis. group analysis.
[0370] B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com