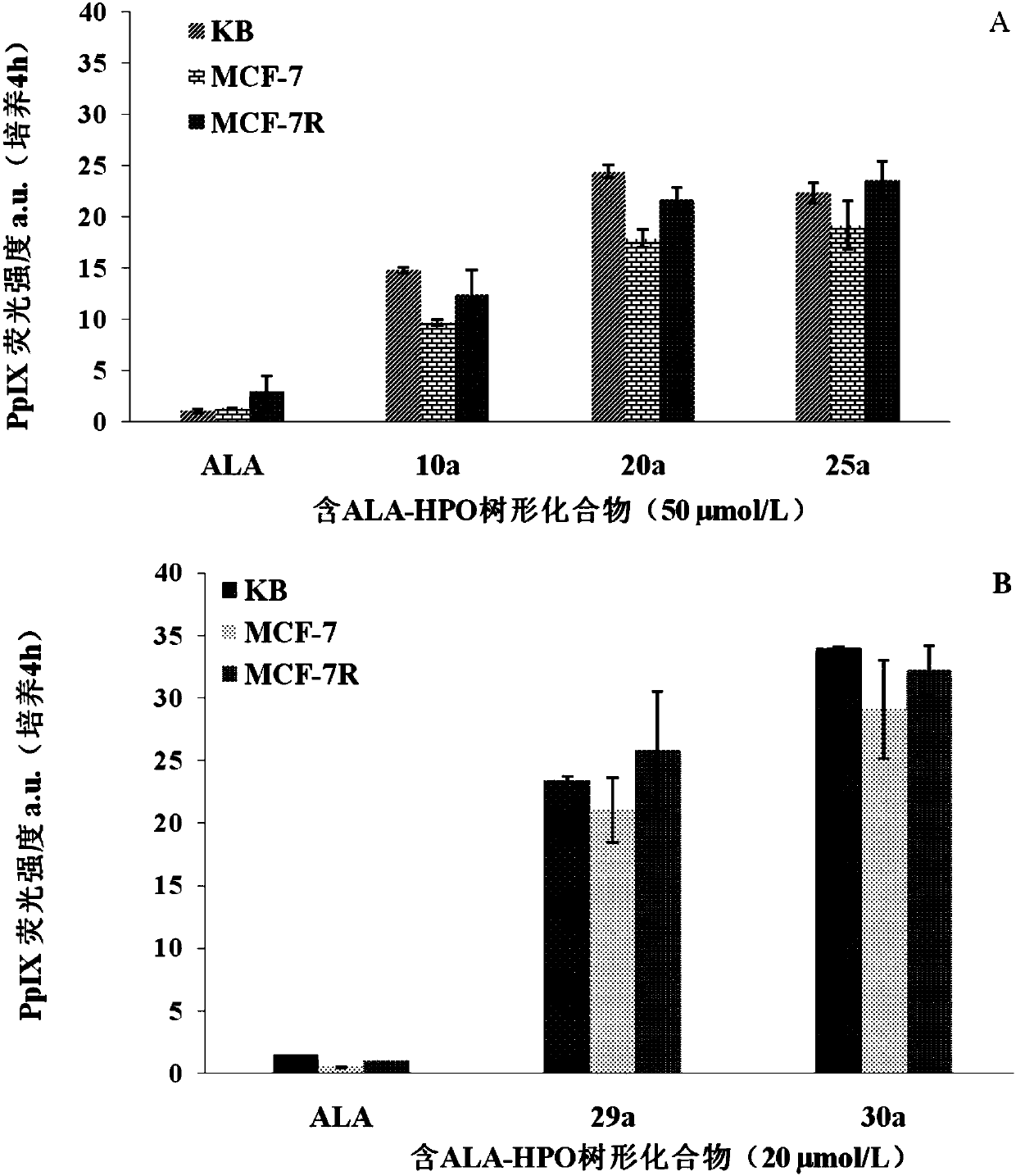
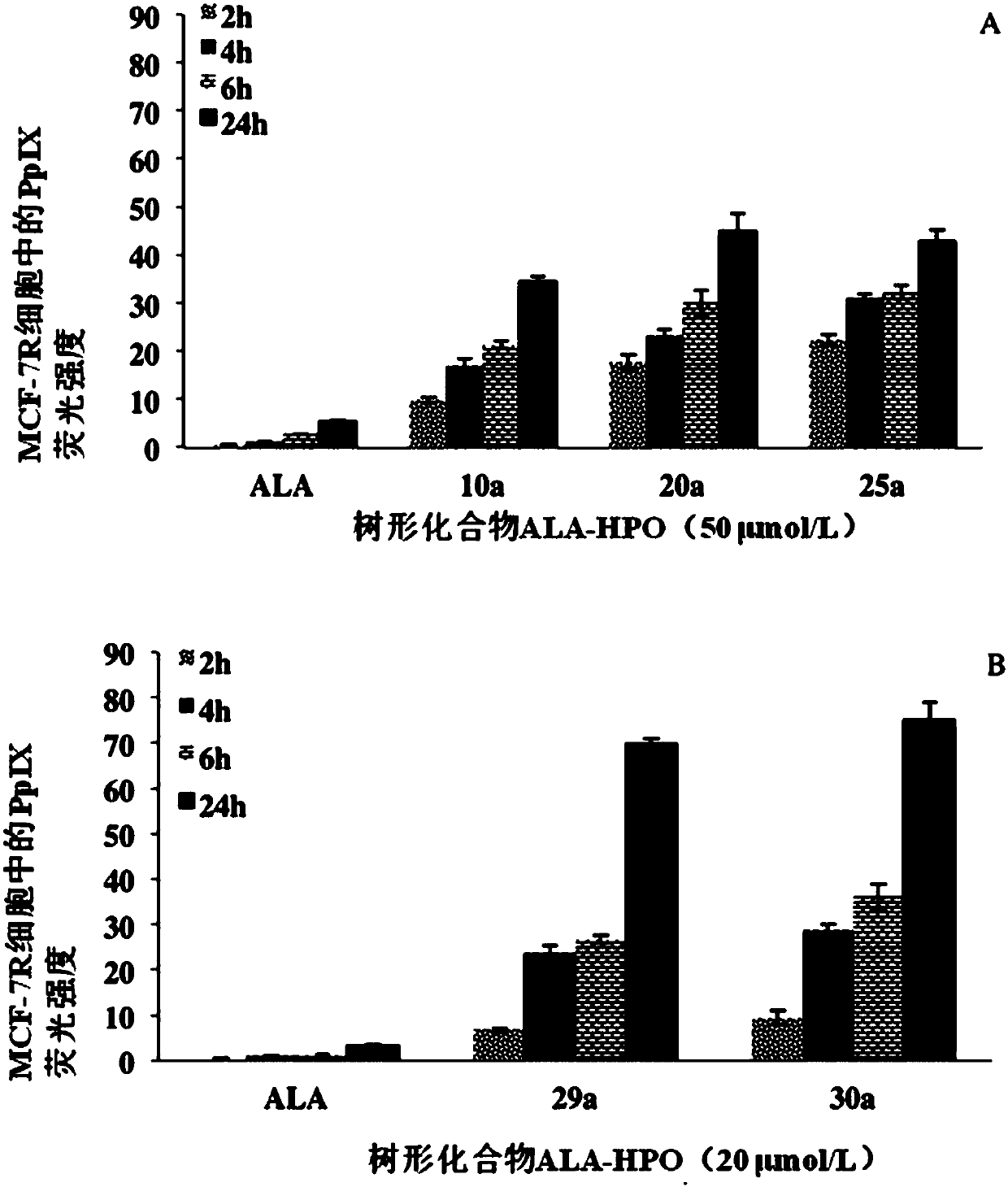
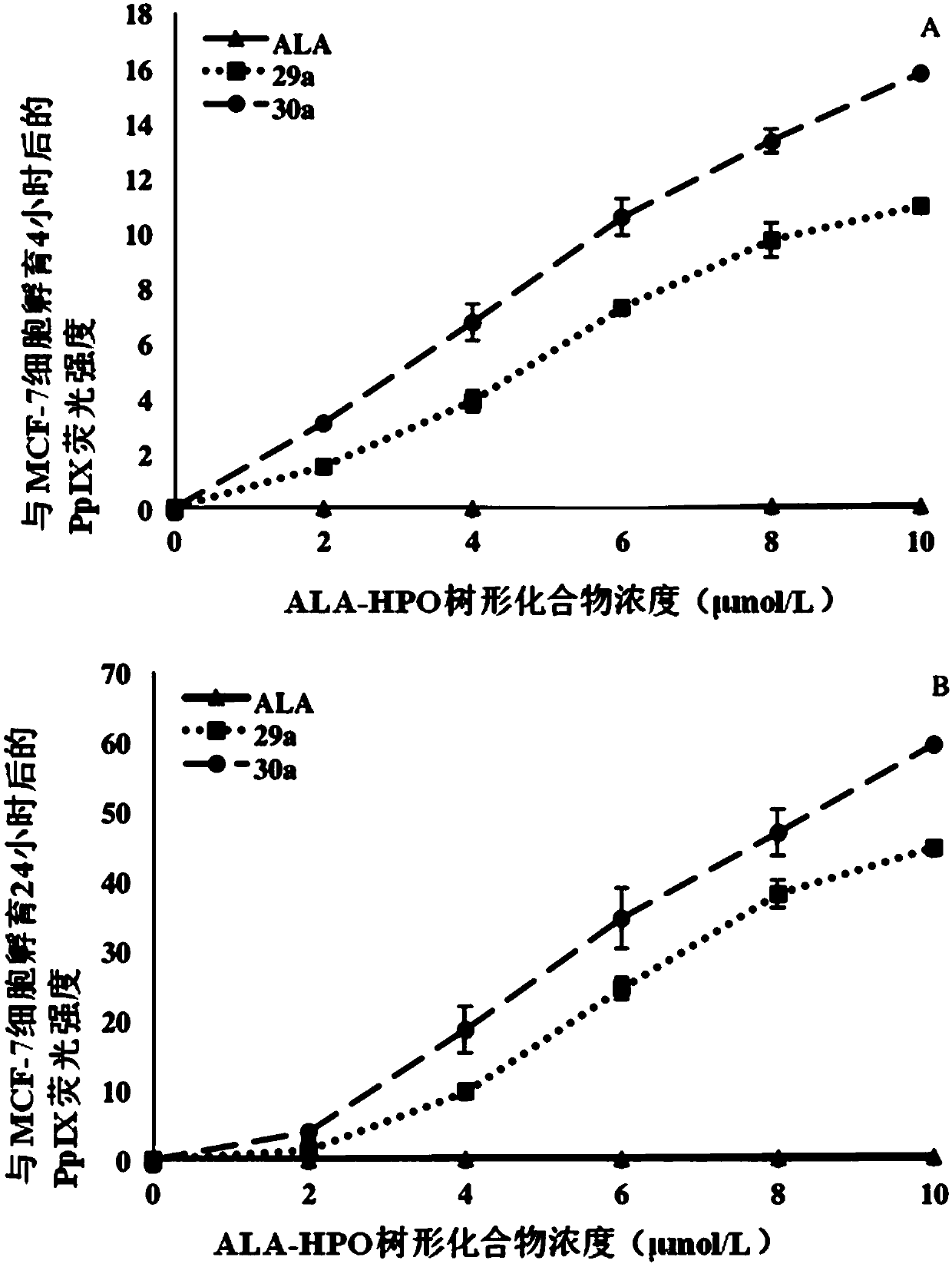
Dendrimer containing 5-aminolevulinic acid and 3-hydroxypyridone, its preparation method and use
A technology of aminolevulinic acid and hydroxypyridine, applied in medical preparations containing active ingredients, organic chemistry, drug combinations, etc., can solve problems that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the preparation of dendrimer 10a
[0040] Its synthetic route is shown in Scheme 1:
[0041]
[0042] Its concrete preparation method, carries out following steps successively:
[0043] Tris(3-acetoxypropyl)nitromethane (2): Methylnitrotripropanol (1) (10g, 42mmol) was dissolved in pyridine (50mL), acetic anhydride (25mL) was added, and the mixture was stirred at room temperature for 18h . 50 mL of water was added to the reaction liquid, stirred for 30 min, and then concentrated to about half volume. Dichloromethane (DCM, 100mL) was added to the residual liquid, followed by 5% Na 2 CO 3 The solution (50 mL) was washed with water (50 mL). After drying over anhydrous sodium sulfate, the solution was concentrated to dryness to obtain oily product 2 (14.2 g, 95% yield). 1 H NMR (CDCl 3 ,270MHz)δ:1.53(m,6H,3CH 2 ),1.97(m,6H,3CH 2 ),2.02(s,9H,3COCH 3 ),4.03(m,6H,3CH 2 ); ESI-MS: m / z 362 ([M+H] + ),384([M+Na] + ).
[0044] Three (3-acetoxypropyl) n...
Embodiment 2
[0053] Embodiment 2, the preparation of dendrimer 20a
[0054] Its synthetic route is shown in Scheme2:
[0055]
[0056] Its concrete preparation method, carries out following steps successively:
[0057] Compound 13: Fmoc-β-alanine (2.21g, 7.1mmol), 4-(2-(tert-butoxycarbonyl)ethyl)-4-aminopimelic acid di-tert-butyl ester (amine 12, 3.35g, 8.5mmol), HOBt (1.3g, 8.5mmol), DCC (1.71g, 8.5mmol) were dissolved in anhydrous DMF (30mL), and the mixture was stirred at room temperature overnight. Filtration, the filtrate was concentrated, and the residue was separated and purified by silica gel column chromatography (ethyl acetate / cyclohexane 1:1, V / V), and R f The eluent was 0.42, and the solvent was removed to give the product 13 as a white solid (4.64 g, 92% yield). 1 H NMR (CDCl 3 ,500MHz)δ1.43(s,27H,CH 3 ),1.98(m,6H,CH 2 ),2.21(m,6H,CH 2 ),2.35(m,2H,CH 2 ),3.48(m,2H,CH 2 ),4.21(m,1H,CH),4.35(m,2H,CH 2 ),5.70(br,1H,NH),6.05(s,1H,NH),7.31(m,2H,Ar),7.39(m,2H,Ar),7.60(d...
Embodiment 3
[0066] Embodiment 3, the preparation of dendrimer 25a
[0067] Its synthetic route is shown in Scheme 3:
[0068]
[0069] Its concrete preparation method, carries out following steps successively:
[0070] Compound 21: N-Cbz-L-phenylalanine (10mmol), ALA methyl ester hydrochloride (12mmol), DCC (10mmol), HOBt (10mmol) and [Bmim]Br (10mmol) were dissolved in THF (100mL ). The mixture was stirred at room temperature for 2 hours under nitrogen protection, and then cooled to 0 °C. Diisopropylethylamine (DIPEA) (12 mmol dissolved in 10 mL THF) was added dropwise to the above mixture, and stirred overnight at room temperature. Acetone (100 mL) was added, the precipitate was removed by filtration, and the filtrate was evaporated to dryness. The residue was dissolved in dichloromethane (100 mL), washed successively with 5% dilute hydrochloric acid, saturated sodium bicarbonate and water. It was dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com