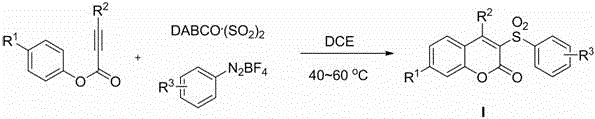
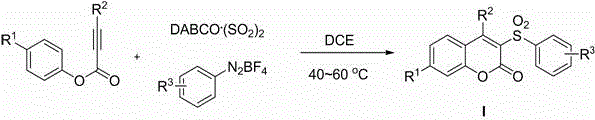
Method for preparing 3-sulfonyl coumarin compound
A technology of sulfonyl coumarin and compounds, which is applied in the field of preparation of 3-sulfonyl coumarin compounds, can solve problems such as bad odor and unfavorable operation and use, achieve low cost, avoid the use of sulfonyl chloride intermediates, and use raw materials easy-to-achieve effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Ia
[0023] Add DABCO to the reaction tube sequentially . (SO 2 ) 2 (2.0 equivalent), phenylpropiolate (0.2 mmol), aryldiazonium salt (1.2 equivalent) and 1,2-dichloroethane (2 mL), under the protection of inert gas nitrogen or argon, in Stir at 40-60°C for 0.5-1.0 hour, until the reaction is complete as detected by TLC, the reaction solution is directly concentrated and separated by column chromatography to obtain the corresponding 3-sulfonylcoumarin compound Ia.
[0024] 1 H NMR (400 MHz, CDCl 3 )δ8.01 (d, J = 8.0 Hz, 2H), 7.57-7.61 (m, 4H),7.50 (t, J = 8.0 Hz,2H), 7.33-7.35 (m, 2H), 7.15 (s, 1H), 7.01 (d, J = 7.6Hz, 1H), 6.90 (d, J = 8.4Hz, 1H), 2.45(s, 3H); 13 C NMR (100 MHz, CDCl 3 ) for 22 h 17 o 4 S(M + +H):377.0842, found: 377.0830.
Embodiment 2
[0026] Ib
[0027] Add DABCO to the reaction tube sequentially . (SO 2 ) 2 (2.0 equivalent), phenylpropiolate (0.2 mmol), aryldiazonium salt (1.2 equivalent) and 1,2-dichloroethane (2 mL), under the protection of inert gas nitrogen or argon, in Stir at 40-60°C for 0.5-1.0 hours, until the reaction is complete as detected by TLC, the reaction solution is directly concentrated and separated by column chromatography to obtain the corresponding 3-sulfonylcoumarin compound Ib.
[0028] 1 H NMR (400 MHz, CDCl 3 )δ8.02 (d, J = 7.6 Hz, 2H), 7.58-7.64 (m, 2H),7.51 (t, J = 7.6 Hz,2H), 7.40 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 8.4 Hz, 1H),7.18-7.26 (m, 3H), 7.09 (d, J = 8.0Hz, 1H), 2.51(s, 3H); 13 C NMR (100 MHz, CDCl 3 ) δ 159.9, 155.5, 153.8, 140.2, 139.2, 134.5, 133.5, 129.9, 129.4, 129.0, 128.8, 128.5, 127.4, 125.8, 124.7, 120.3, 116.7cd for Cd MS 21.5 22 h 17 o 4 S(M + +H): 377.0842, found: 377.0841.
Embodiment 3
[0030] IC
[0031] Add DABCO to the reaction tube sequentially. (SO 2 ) 2 (2.0 equivalent), phenylpropiolate (0.2 mmol), aryldiazonium salt (1.2 equivalent) and 1,2-dichloroethane (2 mL), under the protection of inert gas nitrogen or argon, in Stir at 40-60°C for 0.5-1.0 hours until the reaction is complete as detected by TLC. The reaction solution is directly concentrated and separated by column chromatography to obtain the corresponding 3-sulfonylcoumarin compound Ic.
[0032] 1 H NMR (400 MHz, CDCl 3 )δ 8.00 (d, J = 7.6 Hz, 2H), 7.60-7.67 (m, 2H),7.52 (t, J = 8.0 Hz, 2H), 7.30-7.37 (m, 5H), 7.23 (t, J = 8.0 Hz, 1H), 7.05(d, J= 8.0 Hz, 1H); 13 C NMR (100 MHz, CDCl 3 ) δ 163.2 (d, 1 J F = 248.3), 158.5,155.3, 153.8, 140.0, 134.8, 133.7, 129.6 (d, 2 J F =21.5 Hz), 129.5, 129.0,128.6, 128.2, 126.2, 124.9, 120.0, 116.9, 115.5 (d, 2 J F =22.0 Hz); HRMS calcd for C 21 h 14 FO 4 S(M + +H): 381.0591, found: 381.0588.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com