A kind of boron-doped garnet type llzo lithium ion conductor and preparation method thereof
A garnet-type, lithium-ion technology, applied in electrical components, electrochemical generators, electrolyte immobilization/gelation, etc., can solve the problem of not showing boron ions, etc., and achieve good electrochemical stability and high ionic conductivity. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
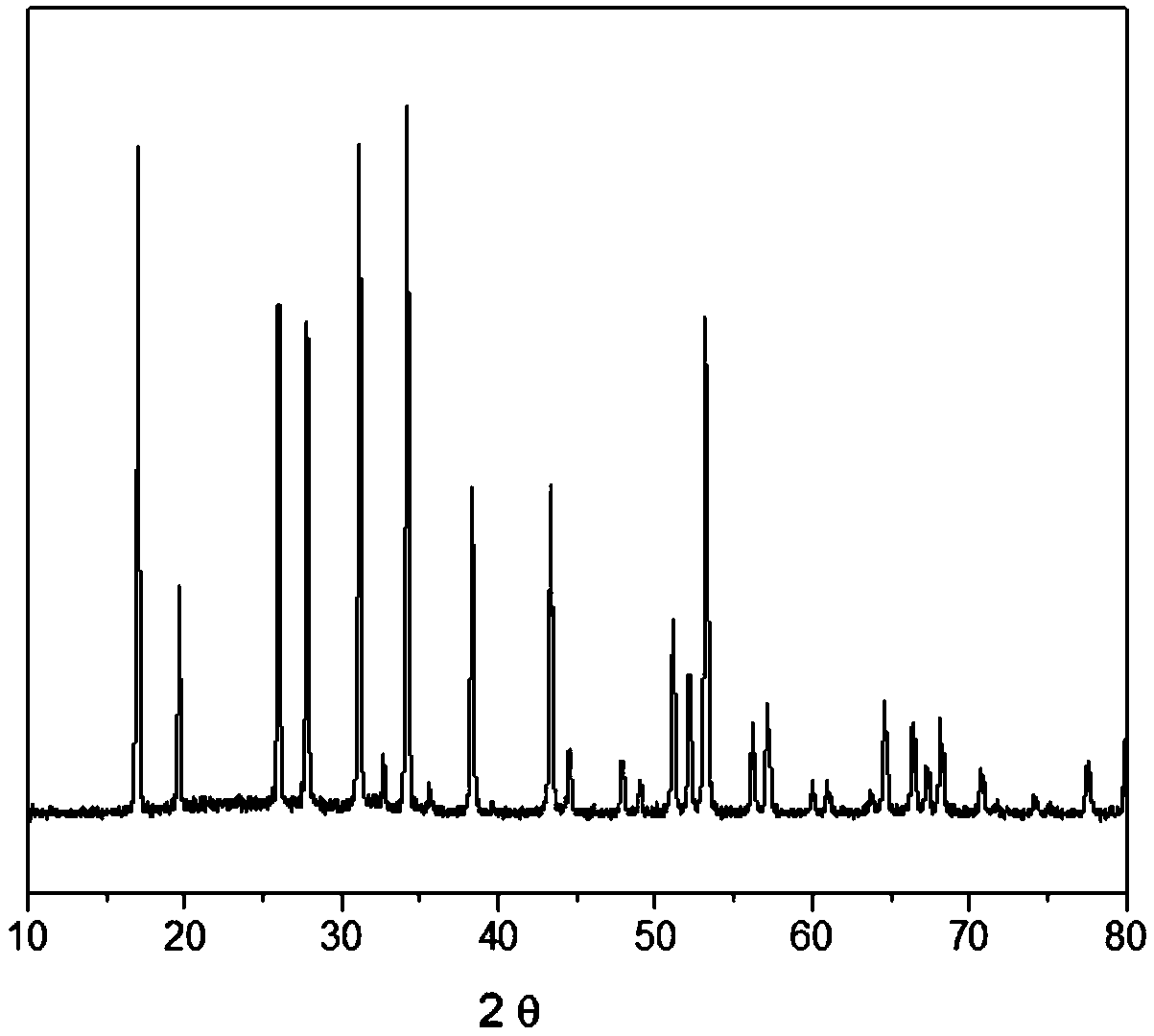
[0020] With LiOH, La(NO 3 ) 3 、HBO 2 and ZrO(NO 3 ) 2 As raw material, its molar ratio is LiOH: La(NO 3 ) 3 :HBO 2 :ZrO(NO 3 ) 2 =6.8:2.8:0.2:2.0. Dissolve the raw materials in water to make a solution, then mix according to the above molar ratio, stir and heat to 80°C; put the evenly stirred solution into a microwave reaction vessel; seal the reaction vessel, set the microwave to heat to 250°C for reaction 2 hours; after natural cooling, the solid phase product was separated, washed and dried to obtain the precursor powder. The above precursor powder was formed into a disc with a diameter of 2.0 cm and a thickness of 0.3 cm by cold isostatic pressing at 30 MPa, and then sintered in air at 1180 ° C under normal pressure. After 4 hours of heat preservation, it was naturally cooled to room temperature to obtain a dense ceramic.
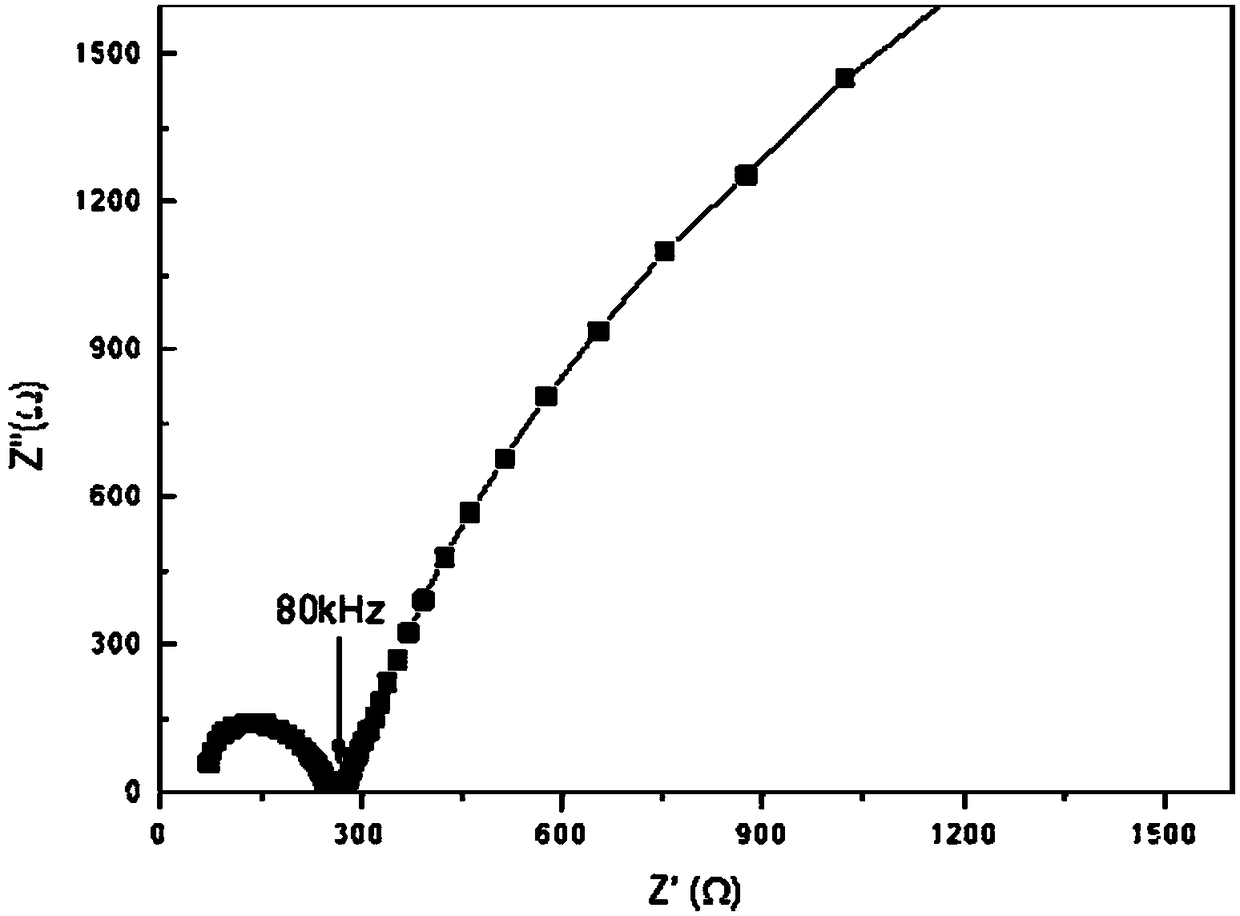
[0021]The diameter of the ceramic sample obtained in Example 1 is about 1.8 cm, the thickness is about 0.18 cm, and the gold film is used as ...
Embodiment 2
[0023] Water-soluble LiNO 3 , La(NO 3 ) 3 、H 3 BO 3 and ZrO(NO 3 ) 2 As raw material, its molar ratio is LiNO 3 :La(NO 3 ) 3 :H 3 BO 3 :ZrO(NO 3 ) 2 =7.2:2.4:0.6:2.0. Mix the raw materials according to the above molar ratio, add water, stir and heat to 80°C, stir the solution evenly, and put it into a microwave reaction vessel; seal the reaction vessel, set the microwave to heat to 250°C, and react for 3 hours; after natural cooling, The solid phase product is separated, washed and dried to obtain the precursor powder. The above precursor powder was formed into a disc with a diameter of 2.0 cm and a thickness of 0.3 cm by cold isostatic pressing at 30 MPa, and then sintered in air at 1120 ° C under normal pressure. After 6 hours of heat preservation, it was naturally cooled to room temperature to obtain a dense ceramic.
[0024] The diameter of the ceramic sample obtained in Example 2 is about 1.8 cm, the thickness is about 0.18 cm, and the gold film is used as a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com