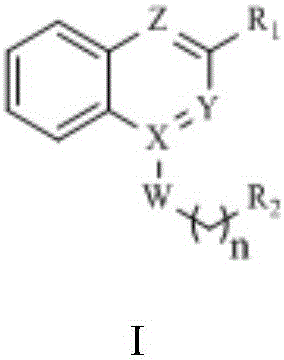
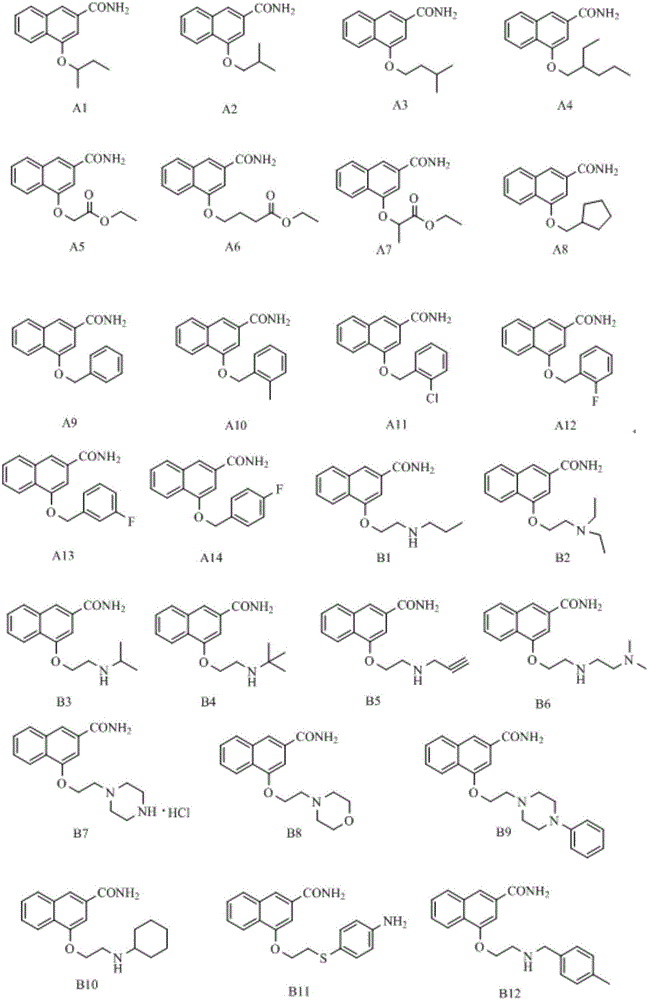
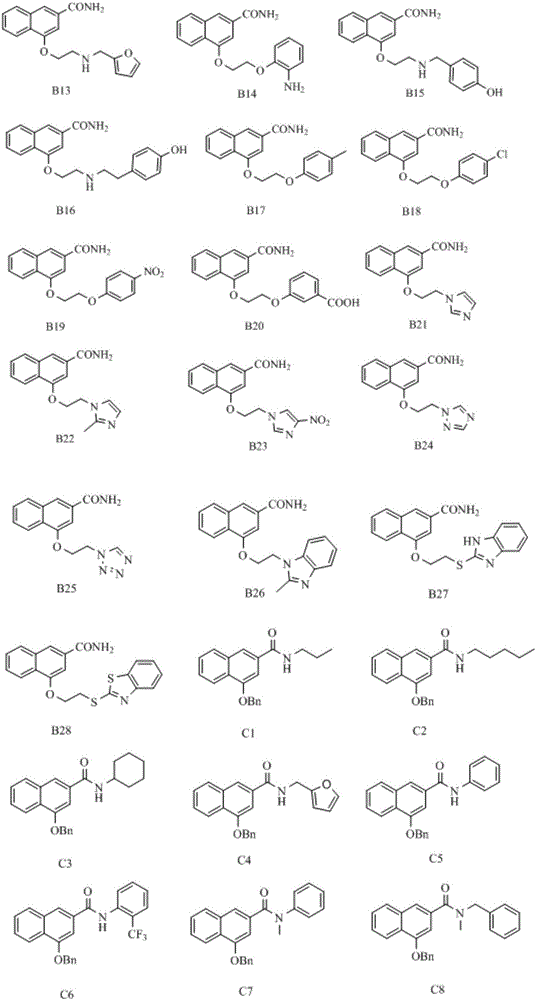
Disubstituted bicyclic derivative and application thereof as efflux pump inhibitor to anti-microbial
A kind of technology of reaction and compound, applied in the field of disubstituted bicyclic derivatives and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Preparation of benzylidene succinic acid (compound of formula 2)
[0096] Benzaldehyde (15.0g, 141mmol) and dimethyl succinate (25.8g, 17.6mmol) were dissolved in ethanol (100mL), and the above mixture was slowly added dropwise to ethanol ( 150mL) solution, reflux for 4h. After the reaction was completed, evaporated to dryness under reduced pressure, the resulting solid was dissolved in 15% NaOH aqueous solution (100 mL), heated to reflux for 3 h, the reaction solution was cooled to room temperature, extracted once with ethyl acetate (60 mL), and the aqueous phase was retained. Use concentrated HCl to adjust the pH to <2, and a white solid precipitates out, which is filtered with suction, and the filter cake is washed twice with water. The filter cake was dried to obtain 21.75 g of white solid, with a yield of 75%.
Embodiment 2
[0098] Preparation of 4-hydroxyl-2-naphthoic acid (compound of formula 3)
[0099] Benzylidene succinic acid (20.6g, 100mmol) was slowly added to stirred concentrated sulfuric acid (147g, 1500mmol) at low temperature, protected by nitrogen, and then transferred to room temperature for 48h. The reaction solution was slowly added dropwise into stirred ice water (300 mL), and a yellow solid was precipitated. Suction filtration with a Buchner funnel, washing with ice water three times to obtain a crude brown-yellow solid. Finally, it was recrystallized with ethanol / water to obtain 12.2 g of a yellow solid with a yield of 65%.
Embodiment 3
[0101] Preparation of 4-acetoxy-2-naphthoic acid (compound of formula 4)
[0102] 4-Hydroxy-2-naphthoic acid (12.2g, 65mmol) was dissolved in pyridine (80mL), and acetic anhydride (26.5g, 260mmol) was slowly added dropwise at room temperature, and reacted at room temperature for 3h. After the reaction was completed, evaporate to dryness under reduced pressure, add water (120 mL) to the obtained residue, adjust the pH=3 with 1N HCl, extract three times with ethyl acetate (3×80 mL), combine the organic phases, wash the organic phases with saturated brine, Anhydrous Na 2 SO 4 After drying, filtering, and evaporating to dryness under reduced pressure, 13.3 g of brown-yellow solid was obtained, with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com