2,3-Naphthalimide derivatives and their preparation methods and applications
A reaction, methyl technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
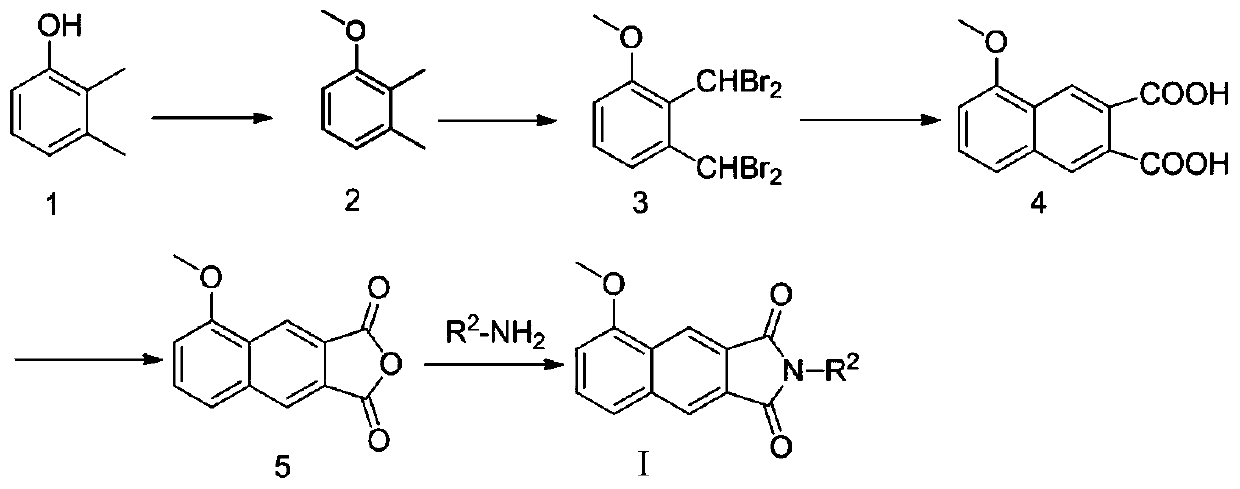
[0102] Example 1 Preparation of α,α,α′,α′-Tetrabromo-3-methoxy-o-xylene (Compound 3)
[0103] 2,3-Xylenol (3.00g, 24.6mmol) was dissolved in acetone (30ml), solid anhydrous potassium carbonate (10.0g, 73.8mmol) was added, and methyl iodide (5ml, 73.8mmol) was added under dark conditions ), the mixture was heated to 50°C and stirred at reflux for 24h. After the reaction was detected by thin layer chromatography (TLC), the stirring was stopped, diluted with 100ml of water, extracted three times with ethyl acetate (50ml×3), the organic phase was combined, and anhydrous sodium sulfate was added to the organic phase to dry for 12h. Filtration and rotary evaporation to remove the ethyl acetate solvent yielded 3.5 g of the crude product of 3-methoxy-o-xylene (compound 2) as a light yellow oily liquid, with a crude product yield of 104.7%.
[0104] Dilute and dissolve the crude product of 3-methoxy-o-xylene with carbon tetrachloride (100ml), add N-bromosuccinimide (NBS, 34.0g, 190....
Embodiment 2
[0105] Example 2 Preparation of 5-Methoxy-2,3-Naphthalic Anhydride (Compound 5)
[0106] α, α, α', α'-tetrabromo-3-methoxy-o-xylene (1.88g, 4.2mmol), maleic anhydride (0.41g, 4.2mmol) and potassium iodide prepared in Example 1 The solid (4.83g, 30mmol) was put into N,N-dimethylformamide (DMF, 20ml), stirred evenly, and the reaction system was heated to 70°C and stirred for 8h. TLC detects that the reaction is substantially complete, cooling stops stirring, and the reaction solution is poured into a pre-prepared dilute sodium bisulfite solution (5g / 200ml), and it is observed that a yellow solid is precipitated immediately, and left standing for a period of time. Filter, wash the filter cake three times with a small amount of dilute sodium bisulfite solution, take the filter cake and dry it to obtain 0.63 g of crude yellow solid of 5-methoxy-2,3-naphthalene dicarboxylic acid (compound 4), the yield of crude product is 61% .
[0107] Weigh the crude product of 5-methoxy-2,3-...
Embodiment 3
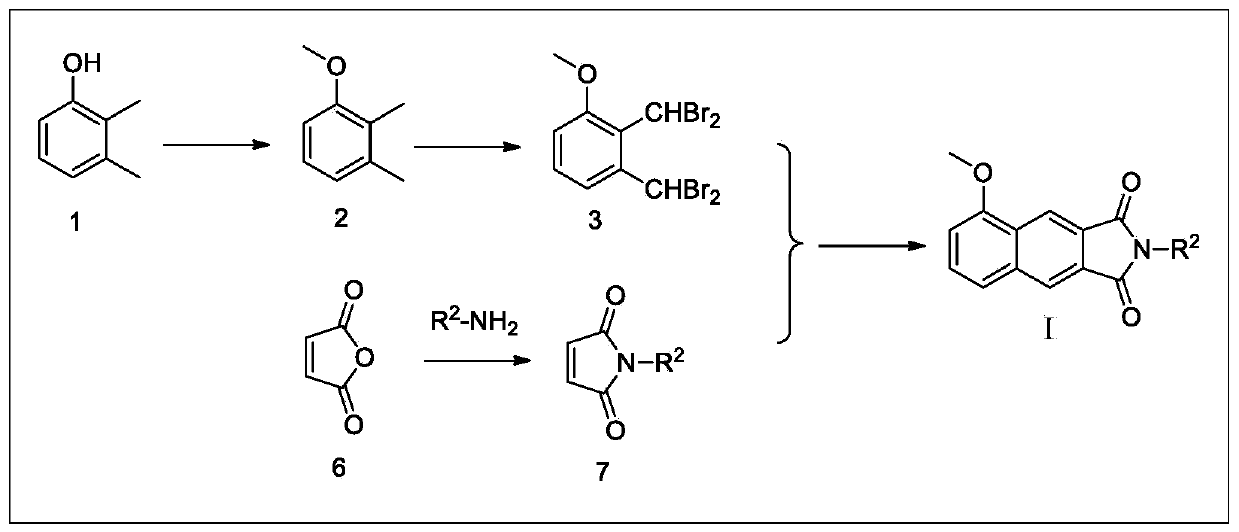
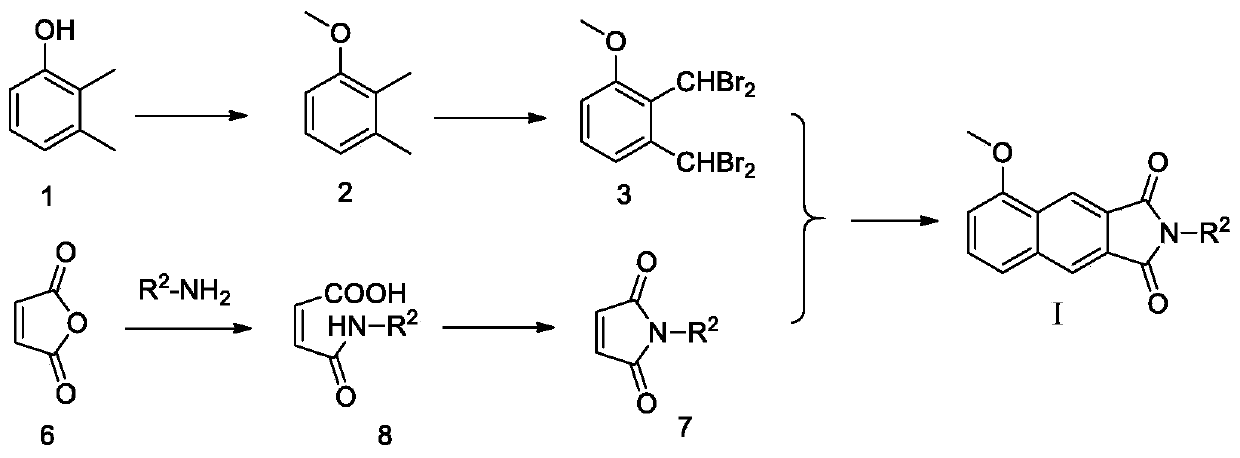
[0108] Example 3 Preparation of N-Benzylmaleimide
[0109] Maleic anhydride (0.98g, 10mmol) was dissolved in glacial acetic acid (15ml), and benzylamine (0.83g, 6.67mmol) was slowly added dropwise with electromagnetic stirring, and the reaction solution was heated to 120°C and stirred at reflux for 4h. The TLC detection reaction is substantially complete, stop stirring, cool to room temperature, dilute the neutralization reaction solution with prepared dilute sodium hydroxide solution (8g / 100ml), extract three times with ethyl acetate (30ml×3), collect the organic phase, The organic phase was dried with anhydrous sodium sulfate for 12h. After filtration, the filtrate was subjected to silica gel column chromatography, and the eluent was ethyl acetate:petroleum ether=1:10 to obtain 0.41 g of a white solid with a yield of 30.0%.
[0110] Wherein, N-m-fluorobenzylmaleimide can be prepared using the same method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com