Oridonin fluorescent probe and its preparation method and use in target cell positioning
A technology of Rubescensin A and fluorescent probes, which is applied in the direction of luminescent materials, biochemical equipment and methods, and the determination/testing of microorganisms, and can solve the incomplete elucidation of the anti-tumor molecular mechanism and molecular effects of Rubescensin A. The mechanism needs to be explored, the target of cell action and the mode of action are not clear, etc., to achieve the effect of mild conditions and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of Oridonin A Fluorescent Probe
[0037] Compound a (1g, 3.83mmol, 1equiv.) and 1,4-butanediol (3.45g, 38.27mmol, 10equiv.) were dissolved in dichloromethane (20.0mL), stirred at room temperature for 18h, concentrated and added to water, Extract with ethyl acetate (30mL×3), combine the organic layers, wash with water, wash with saturated brine, anhydrous Na 2 SO 4 After drying, column chromatography (MeOH / CH 2 Cl 2 1:100, v / v) to obtain the target compound b (1.06 g, 83%). 1 H NMR (300MHz, CDCl 3 ): δ (ppm) 1.23 (t, J = 6.0Hz, 6H), 1.26 (m, 2H), 1.89 (m, 2H), 2.08 (br, 1H), 3.44 (q, J = 6.0Hz, 4H) , 3.73(t, J=6.0Hz, 2H), 4.35(t, J=6.0Hz, 2H), 6.46(d, J=2.4Hz, 1H), 6.61(dd, J=9.0, 2.4Hz, 1H) , 7.36(d, J=9.0Hz, 1H), 8.44(s, 1H); 13 C NMR (CDCl 3 , 75MHz): δ (ppm) 164.0, 158.0, 152.5, 149.0, 130.6, 109.1, 107.2, 96.2, 64.7, 61.5, 44.6, 29.0, 24.5, 12.0; MS (ESI) m / z: 334.3 [M+H] + .
[0038] Dissolve compound b (1g, 3.0mmol, 1equiv.) and glutaric anhydr...
Embodiment 2
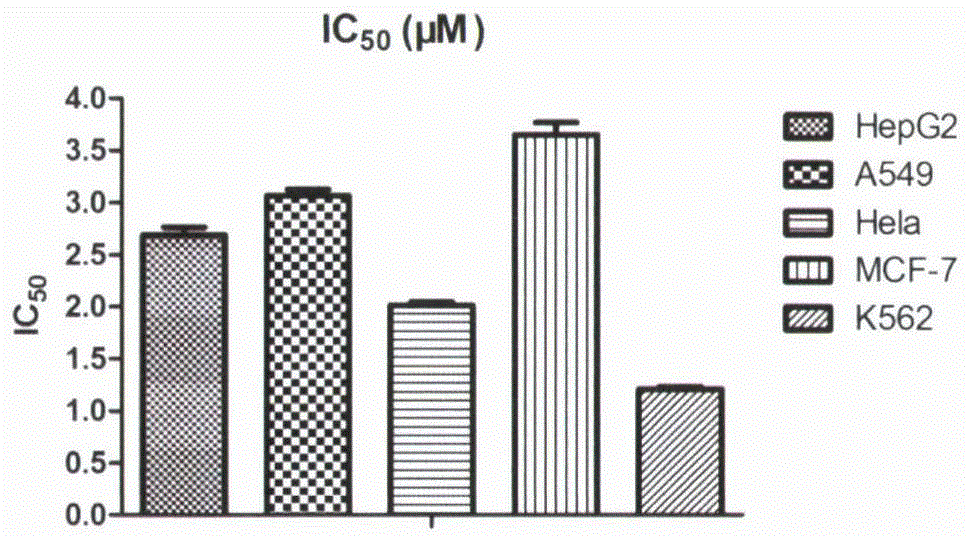
[0041] Antiproliferative Activity of Oridonin A Fluorescent Probe
[0042] The tumor cells in the logarithmic growth phase were digested, counted, and prepared at a concentration of 5×10 4 cells / mL of cell suspension, add 100 μl of cell suspension to each well of a 96-well plate (5×10 per well 3 cells). Place the 96-well plate at 37°C, 5% CO 2 After culturing in the incubator for 24 hours, the drug was diluted to the desired concentration with complete medium, and 100 μL of the corresponding drug-containing medium was added to each well. Place the 96-well plate at 37°C, 5% CO 2 After culturing in the incubator for 72 hours, stain the 96-well plate with MTT; add 20 μL MTT (5 mg / mL) to each well, and continue culturing in the incubator for 4 hours; discard the medium, add 150 μL DMSO to each well to dissolve, and shake gently for 10 minutes. Mix well; λ=490nm, read the OD value of each well with a microplate reader, and calculate the inhibition rate. Experimental results (s...
Embodiment 3
[0043] Example 3 Rubescensin A fluorescent probe staining of HepG2 cells
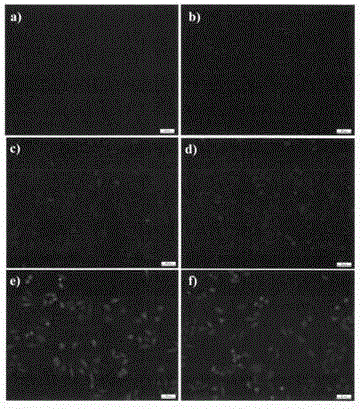
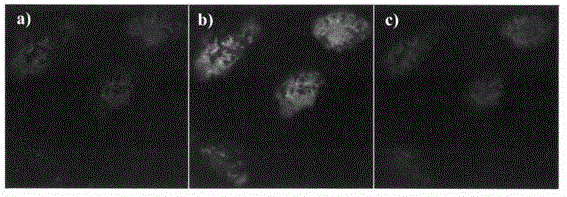
[0044] HepG2 cells in the logarithmic growth phase were selected and seeded on glass coverslips placed in a 6-well plate, 5×10 per well 5 After 24 hours of attachment, compound d (1 μM in DMSO) was added to the complete medium and incubated at different time points (0, 5, 10, 15, 30, 60 minutes). After incubation for the corresponding time, cells were fixed with 4% paraformaldehyde (in 1×PBS) for 10 minutes, and after washing with PBS, the glass coverslips were removed for fluorescence imaging. Under the fluorescent microscope, observe the coloring part of the cells, the distribution of fluorescence and the change of brightness, etc., the results are shown in figure 2 . It can be seen from the figure that the oridonin fluorescent probe can quickly enter the cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com