A biological tissue mass spectrometry imaging method
A mass spectrometry imaging and biological tissue technology, applied in the field of mass spectrometry imaging, can solve problems such as unfavorable optimization of mass spectrometry imaging, inability to regulate mass spectrometry signals, complex sample processing, etc., and achieve the effects of broad application prospects, high sensitivity, and accurate detection results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The biological tissue mass spectrometry imaging method provided in this embodiment includes the following steps:
[0028] (1) Select the isolated mouse kidney tissue, freeze and slice it, and obtain the mouse kidney tissue section;
[0029] (2) Select a corundum rod, attach the mouse kidney tissue section obtained in step (1) to the corundum rod, and let it dry naturally;
[0030] (3) Add the acriflavine solution dropwise on the mouse kidney tissue section, and let it stand to make it spread evenly;
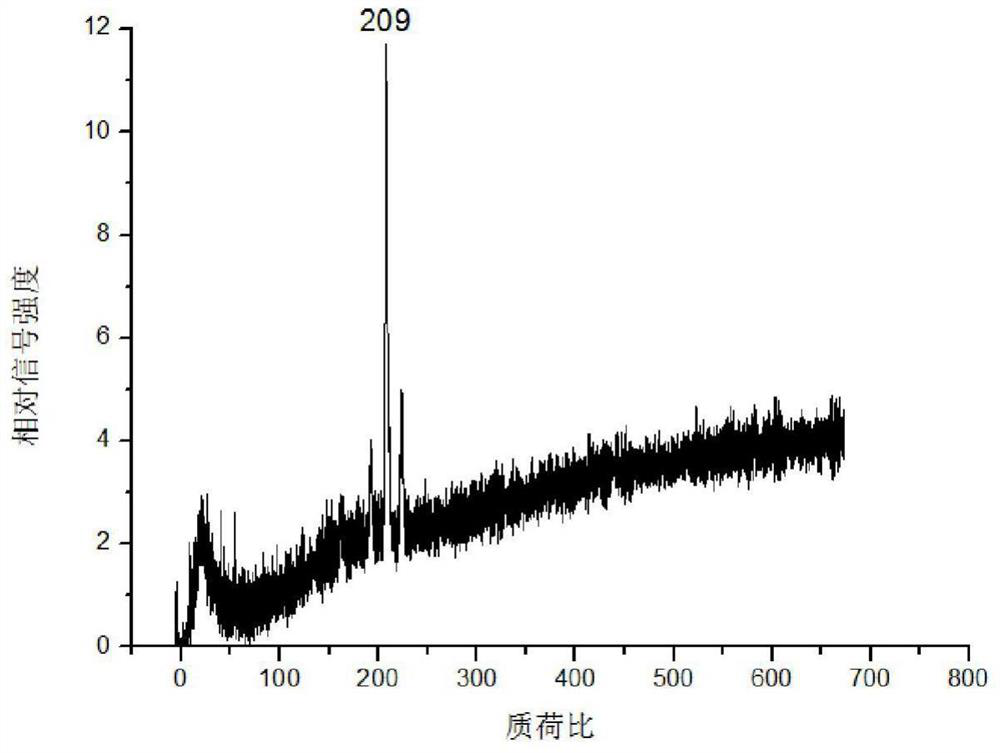
[0031] (4) Fix the corundum rod with the mouse kidney tissue slices on the injection rod of the mass spectrometer, enter the mass spectrometer with laser through the injection system for detection, and obtain the acriflavine in the mouse kidney tissue mass spectrum.
[0032] In the step (1), the mice are selected as Balb / c male mice, 7-8 weeks old.
[0033] The concentration of acriflavine solution in step (3) is 10 -3 g / mL, 10 -3 When the acriflavine solution of g / mL...
Embodiment 2
[0041] The biological tissue mass spectrometry imaging method provided in this embodiment includes the following steps:
[0042] (1) Select the isolated mouse kidney tissue, freeze and slice it, and obtain the mouse kidney tissue section;
[0043] (2) Select a corundum rod, attach the mouse kidney tissue section obtained in step (1) to the corundum rod, and let it dry naturally;
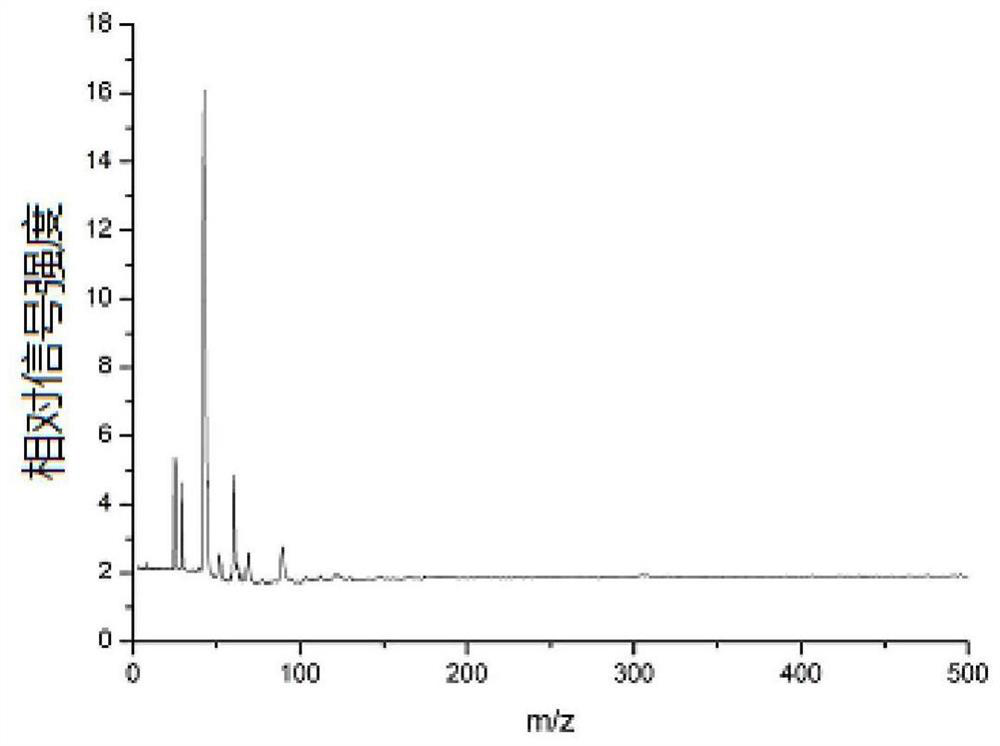
[0044] (3) Fix the corundum rod with the mouse kidney tissue slices on the injection rod of the mass spectrometer, enter the mass spectrometer with laser through the sample injection system for detection, and obtain the acriflavine in the mouse kidney tissue mass spectrum.
[0045] In the step (1), the mice are selected as Balb / c male mice, 7-8 weeks old.
[0046]In step (3), the corundum rod adhered to the mouse kidney tissue slice is fixed on the sample rod of the mass spectrometer, and then enters the mass spectrometer with laser for detection through the sample feeding system. The specific proc...
Embodiment 3
[0049] The biological tissue mass spectrometry imaging method provided in this embodiment includes the following steps:
[0050] (1) Select the isolated mouse kidney tissue, freeze and slice it, and obtain the mouse kidney tissue section;
[0051] (2) Select a corundum rod, attach the mouse kidney tissue section obtained in step (1) to the corundum rod, and let it dry naturally;
[0052] (3) 10 μL of 10 -3 g / mL acriflavine solution, let it stand to make it diffuse evenly;
[0053] (4) Fix the corundum rod with the mouse kidney tissue slices on the injection rod of the mass spectrometer, enter the mass spectrometer with laser through the injection system for detection, and obtain the acriflavine in the mouse kidney tissue mass spectrum.
[0054] In the step (1), the mice are selected as Balb / c male mice, 7-8 weeks old.
[0055] In step (3), stand still for 15-25 minutes to make it spread evenly.
[0056] In step (4), the corundum rod adhered with the mouse kidney tissue sl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com