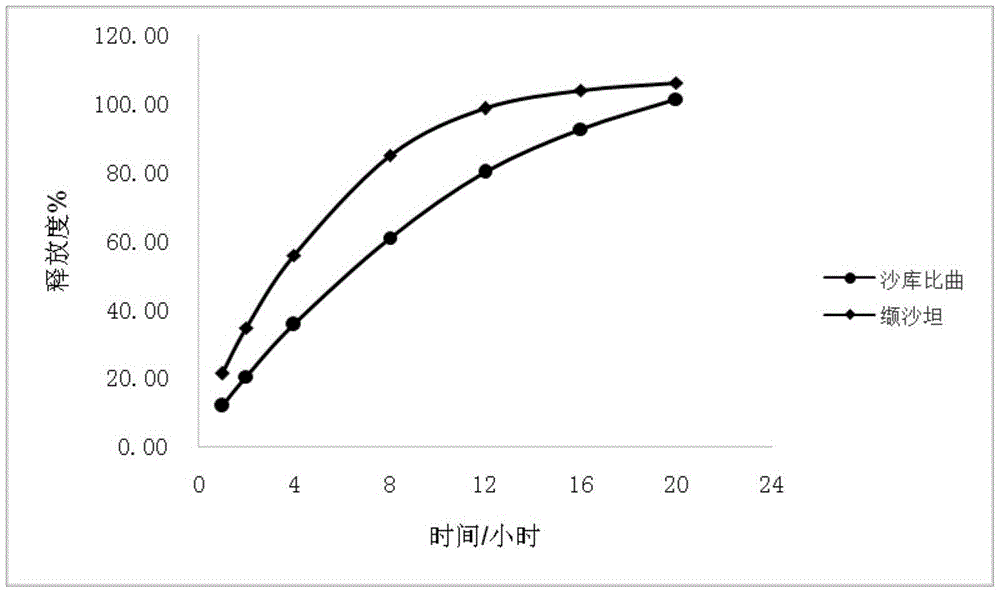
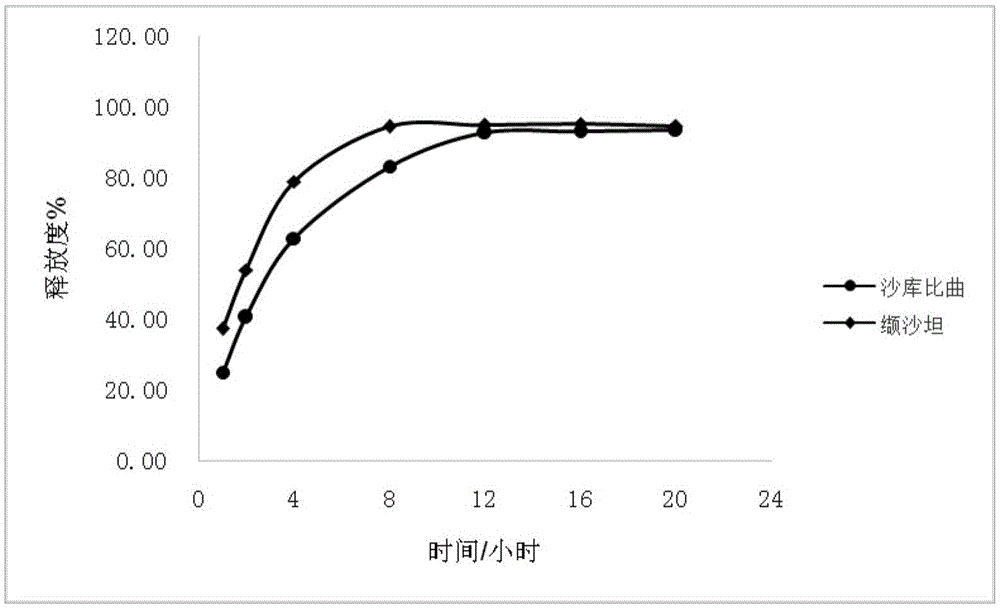
Sacubitril / valsartan sustained release agent and preparation method thereof
A technology of Shakubi and sustained-release preparations, which is applied in pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve peak and valley fluctuations in blood drug concentration, easy adverse reactions, and failure to achieve therapeutic purposes, etc. problems, to achieve the effect of prolonging the drug effect time, reducing toxicity and adverse reactions, and facilitating drug use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The present invention also provides a preparation method of sacubitril-valsartan sustained-release agent, comprising:
[0048] Sacubitril-valsartan co-crystal, hydrophilic gel skeleton material, diluent, first lubricant are mixed, dry granulated, and then the second lubricant is added to mix and tabletted to obtain the sacubitril-valsartan Sartan sustained-release agent;
[0049] The mass ratio of the sacubitril-valsartan co-crystal, skeleton material, diluent to the total amount of the first lubricant and the second lubricant is (10-70): (10-50): (0-80 ): (0.1~10).
[0050] Above-mentioned obtained is sacubitril valsartan sustained-release tablet core material.
[0051] The first lubricant and the second lubricant are added in batches, and the proportion of the lubricant is not particularly limited in the present invention, and can be added according to actual conditions.
[0052] When the sacubitril-valsartan slow-release agent has a coating, the steps also include...
Embodiment 1~3
[0057] According to the proportioning in Table 1, sacubitril valsartan sustained-release preparation is prepared:
[0058] Table 1 Embodiment 1~3 raw material ratio
[0059]
[0060]
[0061] in,
[0062] 49 / 51mg / tablet: refers to that in the sacubitril / valsartan sustained-release preparation, the sacubitril content is 49 mg / tablet, and the valsartan content is 51 mg / tablet. And so on for the rest.
[0063] Preparation:
[0064] Mix sacubitril valsartan co-crystal, hypromellose K15M, lactose and talc powder in a three-dimensional kinematic mixer evenly, then add magnesium stearate and mix, and use the obtained mixed powder with a dry granulator The pressure roller of about 30KN is pressed into strips, and then crushed and granulated by a granulator to obtain the internal phase particles; the internal phase particles, the added part of talc powder and magnesium stearate are placed in a three-dimensional motion mixer and mixed evenly, and then use a high-speed A rotary...
Embodiment 4~6
[0066] According to the proportioning in Table 2, sacubitril valsartan sustained-release preparation is prepared:
[0067] Table 2 Embodiment 4~6 raw material ratio
[0068]
[0069] Preparation:
[0070] Mix sacubitril, trivalsartan co-crystal, hypromellose K100M and talcum powder in a three-dimensional motion mixer evenly, then add magnesium stearate and mix, and use a dry granulator to obtain the mixed powder with a pressure of about 30KN The pressure roller is pressed into strips, and then crushed and granulated by a granulator to obtain the internal phase granules; the internal phase granules, the added part of talcum powder and magnesium stearate are placed in a three-dimensional motion mixer and mixed evenly, and then the high-speed rotary press is used to Tablet machines compress the final blend into tablets. Opadry AMB type moisture-proof coating material is used for coating to obtain moisture-proof coated sustained-release tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com