A Recombinant Escherichia coli Efficiently Transforming Fumaric Acid into L-Asparagine and Its Construction Method and Application
A technology of recombinant Escherichia coli and fumaric acid, applied in the field of bioengineering, can solve the problems of low product yield, large pollution, difficult wastewater treatment, etc., and achieve the effects of improving conversion yield, reducing production cost, and low fumarase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
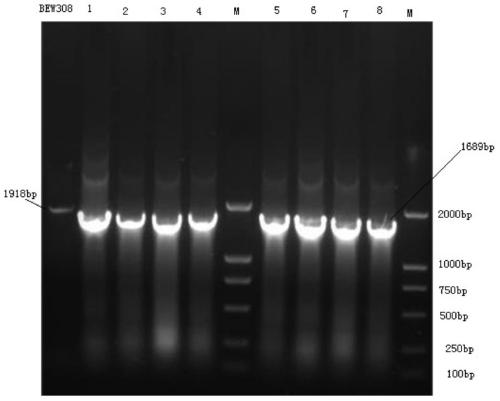
[0032] This example illustrates the process of knocking out the fumarase fumB gene in the parental ammonium-resistant Escherichia coli BEW308 by using homologous recombination technology to obtain an apramycin-resistant strain.
[0033] (1) Using LB medium, cultivate Escherichia coli BEW308 to OD at 37°C under aerobic conditions 600 =0.4~0.6, prepared as electrotransfer competent;
[0034] (2) The pKD46 plasmid was electrotransformed into competent Escherichia coli BEW308. The electric shock conditions are: 200Ω, 25μF, electric shock voltage 2.3kv, electric shock time 4-5ms. Immediately after the electric shock, the bacteria were added to pre-cooled 1mL SOC medium, cultured at 150r / min, 30°C for 1h, and then spread on the LB medium plate with ampicillin (amp) to screen out the positive transformant BEW308 (pKD46);
[0035] (3) Adding 10 mM L-arabinose to LB medium, inducing plasmid pKD46 to express λ recombinase at 30°C to make electroporation competent;
[0036] (4) Using ...
Embodiment 2
[0046] Example 2: In this example, the effect of mutation on the L-aspartase gene (aspC) of Escherichia coli K12 on the enzyme activity was investigated. The specific operation process is: based on the original aspC, the 236 and 249 amino acids are mutated (Lys236Asn, Gly249Thr), and the gene is synthesized by artificial synthesis, named aspC2. The mutated gene was connected to the pTrc99a expression plasmid and introduced into BEW308 (△fumB), and BEW308 (△fumB, aspC2) was constructed to compare with the mutated recombinant strain BEW308 (△fumB, aspC). The results are shown in Table 1:
[0047] Table 1 Changes in enzymatic properties of aspartase before and after mutation
[0048] enzyme specific enzyme activity optimum reaction temperature optimum reaction pH ASPC 38090 37℃ 8.2 ASPC2 45500 37℃ 7.8
Embodiment 3
[0050] This example illustrates the use of homologous recombination to further knock out the fumarase fumAC gene in Escherichia coli BEW308 (ΔfumB), and introduce mutated highly active L-aspartase and L-asparaginase genes.
[0051] The whole experimental operation process is consistent with Example 1, only the homologous sequence is different.
[0052] (1) In this example, the L-aspartase gene (aspC) of Escherichia coli K12 is used as the starting sequence, and the 236 and 249 amino acids are mutated to obtain aspC2, namely Lys236Asn, Gly249Thr, and at the same time upstream of the initiation codon ATP A signal peptide sequence has been added: atgttgaatccgaaggttgcctacatggtctggatgacgtgcctgggtttaacgttgcccagccaggca (shown in SEQ ID NO: 3), the stop codon has been deleted downstream; the coding L-asparaginase gene (asnA), its start codon and L-aspartate There is a 30bp connection sequence between the end genes of the acidase gene to ensure that the two enzymes have high activity a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com