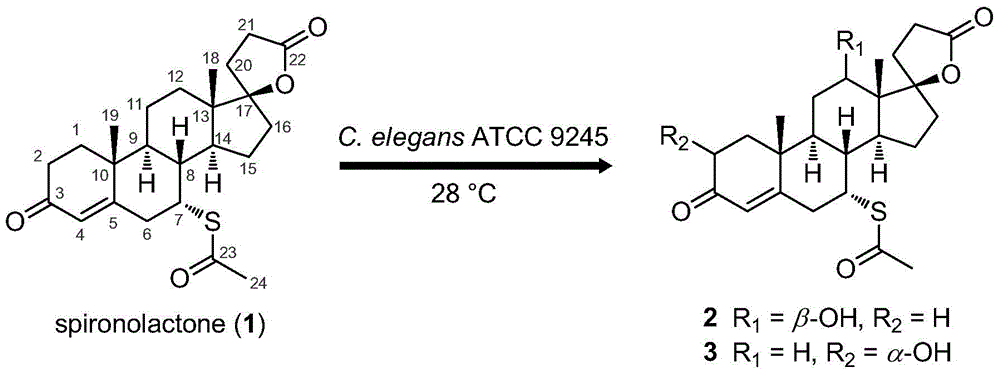
A kind of spironolactone derivative and its microbial conversion preparation method and application
A microbial transformation, spironolactone technology, applied in biochemical equipment and methods, microorganism-based methods, microorganisms, etc., to achieve the effects of high conversion yield, easy large-scale cultivation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of Biocatalyst
[0033](1) Bacterial activation culture: Cunninghamella elegans ATCC9245 (purchased from American Type Culture Collection) was inoculated into potato agar medium, and cultured at 28°C for 3 days to obtain the spores of the fungus. The potato agar (PDA) medium (purchased from EMD Millipore Chemicals in the United States) was dissolved in distilled water according to the instructions, and sterilized by autoclaving at 121°C for 15min;
[0034] (2) Seed expansion culture: Dip the spores from the activated plate in step (1) with a cotton swab and transfer it to a conical flask containing 50 mL of seed medium, and culture at 28 °C and 250 r / min constant temperature shaking for 3 day to obtain seed liquid. The seed medium was potato liquid (PDB) medium (purchased from EMD Millipore Chemicals, USA), dissolved in distilled water according to the instructions, 50mL was divided into a 250mL conical flask, and sterilized by high pressure stea...
Embodiment 2
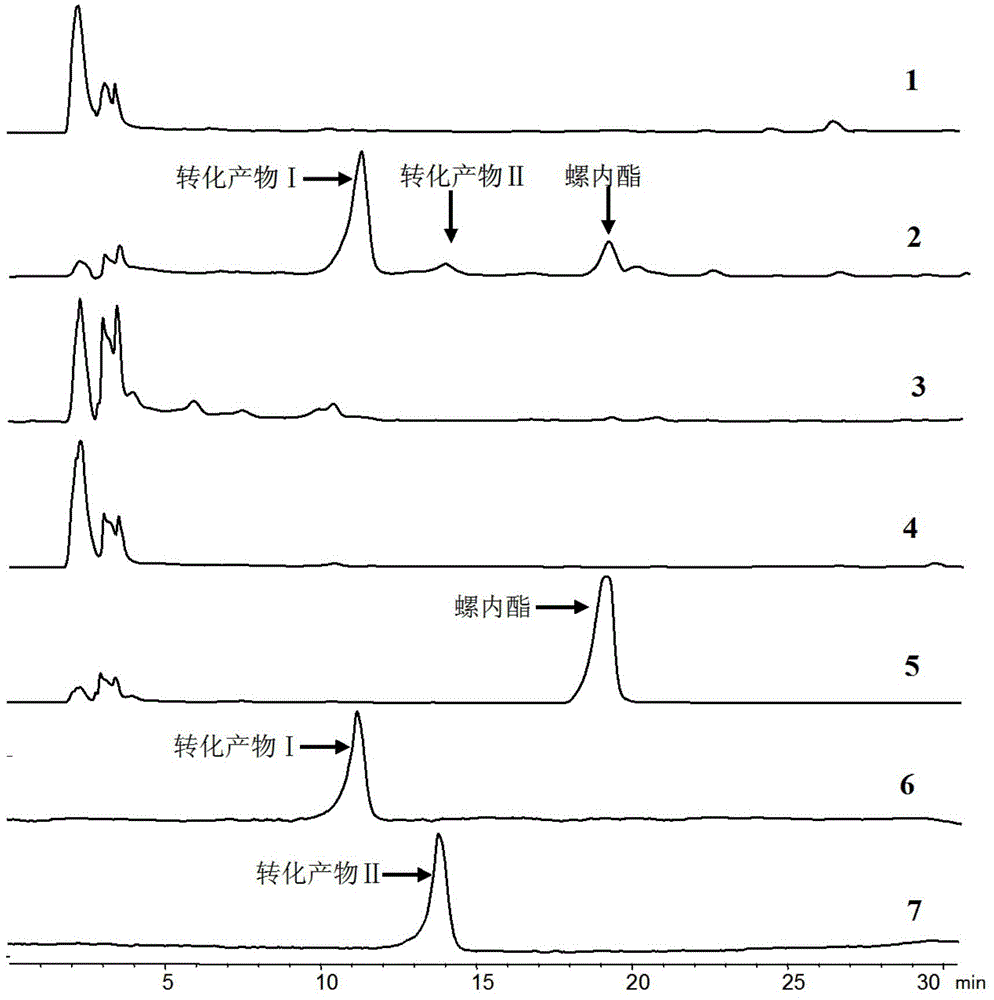
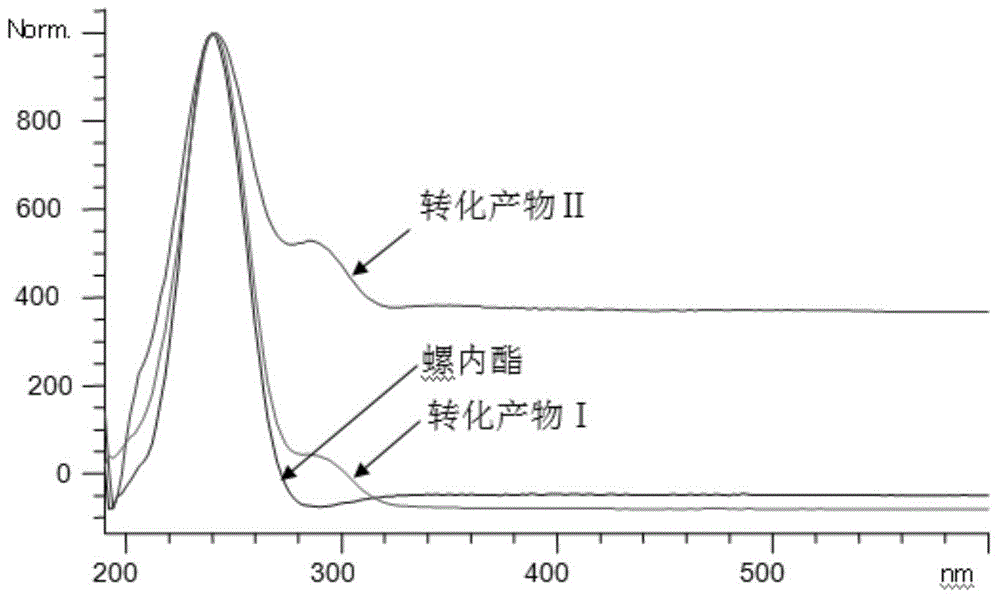
[0036] The tracking analysis of the biotransformation product of embodiment 2 spironolactone
[0037] Take 50 mL of bacterial cell-containing fermentation broth prepared by the method of Example 1, and 1.64 g of wet bacterial cells; 1 mg of spironolactone was dissolved in 0.1 mL of anhydrous ethanol, and then added to the fermentation broth to form a reaction system (the total volume was 50mL), incubate at 28°C and 250r / min for 4 days of constant-temperature shaking transformation. After the transformation is completed, filter the cells with gauze to separate the cells from the culture solution, and use 50mL of anhydrous methanol at 25°C and 100KHz for the cells. Ultrasonic leaching was performed for 30 min, the bacteria were removed by filtration, and the filtrate was evaporated to dryness under reduced pressure with methanol to obtain a concentrate. The concentrate was dissolved with 0.5 mL of anhydrous methanol and filtered to obtain extract a, which was to be analyzed; the ...
Embodiment 3
[0042] Example 3: Preparation of Biocatalysts
[0043] (1) Bacterial activation culture: Cunninghamella elegans ATCC9245 was inoculated into potato agar (PDA) medium, and cultured at 28°C for 3 days to obtain the spores of the fungus. The PDA medium was dissolved in distilled water according to the instructions, and sterilized by high-pressure steam at 121 °C for 15 min;
[0044] (2) Seed expansion culture: Dip the spores from the activated plate in step (1) with a cotton swab and transfer them to 4 Erlenmeyer flasks containing 50 mL of seed medium, and culture at 28°C and 250r / min constant temperature shaking for 3 day to obtain seed liquid. Described seed culture medium is PDB medium, add distilled water to dissolve according to the instruction, 50mL is divided into 250mL Erlenmeyer flask, 121 ℃ of high pressure steam sterilization 15min;
[0045] (3) Bacterial fermentation culture: the seed liquid prepared in step (2) was sequentially inoculated into 20 bottles containing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com