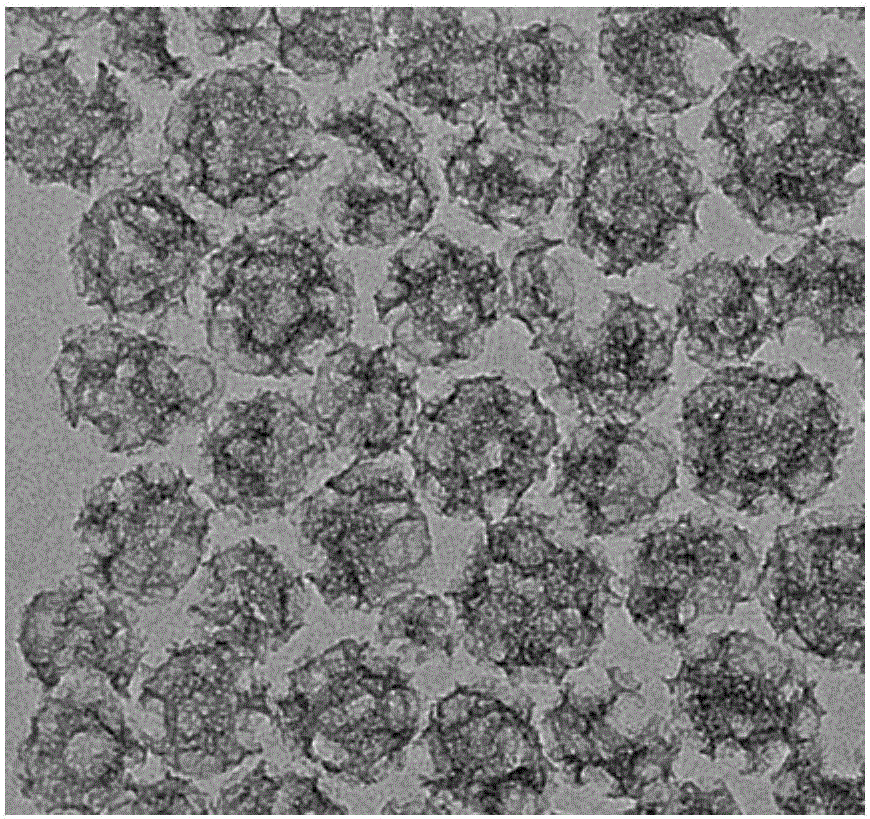
Aperture and particle size monodisperse controllable mesoporous silicon rapid hemostatic powder and preparation method
A hemostatic powder, monodisperse technology, applied in medical formula, medical science, bandages, etc., can solve the problems of inability to achieve hemostatic performance, inability to determine the specific gravity of the hemostatic effect of mesoporous silicon materials, etc., and achieve good biocompatibility and tissue compatibility , Excellent hemostatic effect, no side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A method for preparing mesoporous silicon rapid hemostatic powder with monodisperse and controllable pore size and particle size, comprising the following steps:
[0025] 1) Add 300 mg of cetyltrimethylammonium bromide to 100 ml of water, and purging with high-purity nitrogen (99.99%) for 55 minutes while stirring;
[0026] 2) Add 9ml of n-octane, 5g of methyl methacrylate, 3ml of tetraethyl orthosilicate and azobisisobutylamidine hydrochloride to the liquid obtained in step 1) at 65°C under the stirring condition of 700rpm salt, the final concentration of the azobisisobutylamidine hydrochloride is 0.7 mg / ml, adjust the pH=9 with lysine, react for 6 hours, and then stand still for 16 hours to obtain a milky white suspension;
[0027] 3) Centrifuge the milky white suspension at 12,000 rpm for 20 minutes, and wash it with absolute ethanol for 5 times;
[0028] 4) In an air atmosphere, at a heating rate of 2°C / min, calcined at 600°C for 6 hours to remove the mesoporous te...
Embodiment 2
[0036] A method for preparing mesoporous silicon rapid hemostatic powder with monodisperse and controllable pore size and particle size, comprising the following steps:
[0037] 1) Add 350mg of cetyltrimethylammonium bromide to 100ml of water, and purging with high-purity nitrogen (99.99%) for 65min while stirring;
[0038] 2) Add 4ml of n-octane, 3g of methyl methacrylate, 3.5ml of tetraethyl orthosilicate and azobisisobutylamidine salt to the liquid obtained in step 1) at 70°C under the stirring condition of 800rpm acid salt, the final concentration of the azobisisobutylamidine hydrochloride is 0.8mg / ml, adjust the pH=10 with tris(hydroxymethyl)aminomethane, react for 4h, and then stand for 12h to obtain a milky white suspension;
[0039] 3) Centrifuge the milky white suspension at 16,000 rpm for 10 minutes, and wash it with absolute ethanol for 3 times;
[0040] 4) In an air atmosphere, at a heating rate of 2°C / min, calcined at 700°C for 4 hours to remove the mesoporous te...
Embodiment 3
[0042] A method for preparing mesoporous silicon rapid hemostatic powder with monodisperse and controllable pore size and particle size, comprising the following steps:
[0043] 1) Add 400 mg of cetyltrimethylammonium bromide to 100 ml of water, and purging with high-purity nitrogen (99.99%) for 60 minutes while stirring;
[0044] 2) Add 2ml of n-octane, 1g of methyl methacrylate, 4ml of tetraethyl orthosilicate and azobisisobutylamidine hydrochloride to the liquid obtained in step 1) at 75°C under the stirring condition of 900rpm salt, the final concentration of the azobisisobutylamidine hydrochloride is 0.9mg / ml, adjust the pH=9.5 with lysine, react for 5h, and then stand still for 14h to obtain a milky white suspension;
[0045] 3) Centrifuge the milky white suspension at 14,000 rpm for 15 minutes, and wash it 4 times with absolute ethanol;
[0046] 4) In an air atmosphere, at a heating rate of 2°C / min, calcined at 700°C for 5 hours to remove the mesoporous template, and o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com